What’s the Difference Between Sodium Hydroxide and Potassium Hydroxide for Soapmaking?
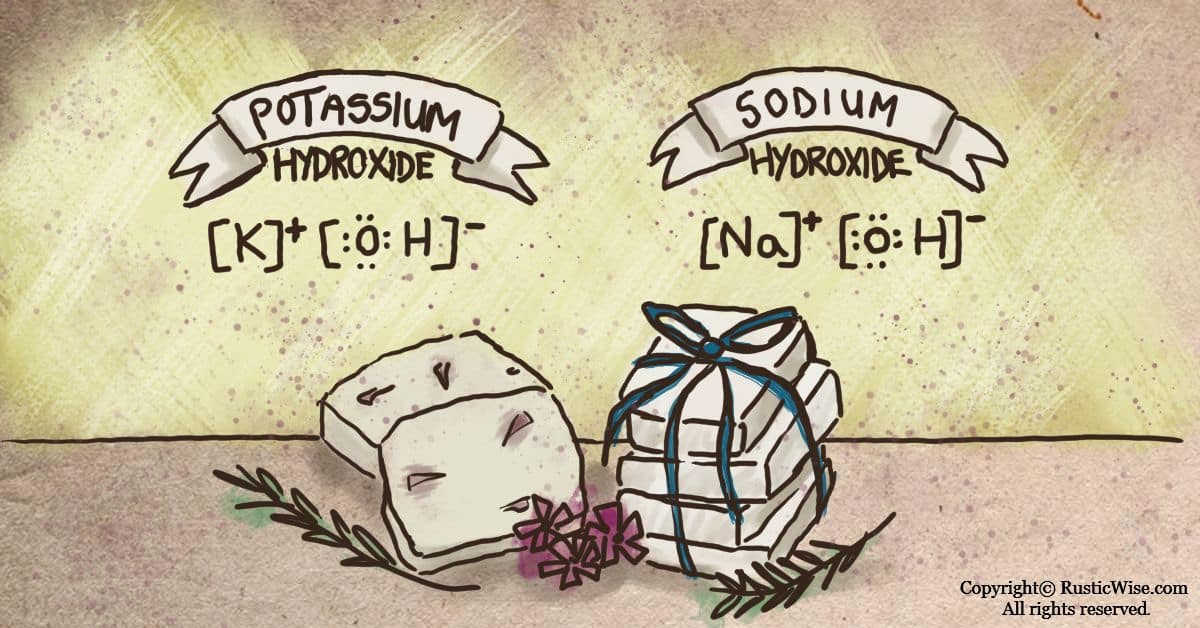
When it comes to soapmaking, there’s some confusion about the difference between sodium hydroxide and potassium hydroxide. Sodium hydroxide (the chemical formula is NaOH) is commonly known as lye, or caustic soda, or soda lye. Potassium hydroxide (KOH) is also called caustic potash. Soaps made using sodium hydroxide are harder (often bar soaps), whereas potassium hydroxide based soaps are more water-soluble, and the results are liquid/gel soaps, or soft soap such as whipped soap, or soap paste.
While they are both key ingredients in soapmaking, there are key differences, and the two cannot be used interchangeably. The confusion stems from the term lye as it’s applied to both sodium hydroxide and potassium hydroxide.
Can you make soft liquid soap with sodium hydroxide, or hard bar soaps with potassium hydroxide? Simply put, no. You would work against the chemical compounds’ natural properties (sodium hydroxide likes to harden, potassium hydroxide does not). You would be better off selecting the appropriate ingredient from the start and save yourself time and effort.
Let’s dive into the similarities and difference between sodium hydroxide and potassium hydroxide as it relates to soapmaking.
Which type of lye to use to make soap?
If you want to get right to the nitty-gritty of sodium hydroxide vs. potassium hydroxide as it pertains to making soap and when to use it, let’s dive right in.
Use sodium hydroxide for making hard bar soaps. The sodium ion helps the soap batter harden up to create cold process or hot process soap bars.
Use potassium hydroxide to make liquid soap, whipped soap, DIY shower gel, soap paste, or other soft soaps. As a general rule, potassium soaps will be softer.

Credit: 123RF.com
Similarities of sodium and potassium hydroxide
If you’re looking into making your own soaps at home, you’ve likely stumbled across products labelled as lye with slightly different scientific names: sodium hydroxide, or potassium hydroxide.
Let’s try to clear things up.
As both chemicals are similar at the molecular level (and both have a hydroxide ion), they have many similar reactions and applications in various industrial applications.
Both sodium and potassium hydroxide are caustic alkalis and metal inorganic compounds which are used to make lathering soaps. When either NaOH or KOH are combined with fat/oil and water, they undergo a chemical reaction known as saponification.
Saponification is simply the conversion of fat and oil molecules into soap (and glycerin as a byproduct).
While both sodium and potassium hydroxide are poisonous and corrosive chemicals on their own (and may cause chemical burns in their raw state), once saponification occurs, they are rendered harmless. That lovely bar of soap you made is now completely safe to use on your skin. (So, if you’re wondering whether sodium or potassium hydroxide is safe to use on skin, the answer is, yes—once the soap has properly saponified.)
Takeaway: You need either sodium hydroxide OR potassium hydroxide in order to make soap. These chemicals kickstart saponification and encourage fats and water to mix. Without one of these two chemicals, you would just be left with a gooey mess.
If you don’t want to handle lye, you can make melt and pour soap, which uses saponified soap bases that you can melt down and pour into molds.
Here are some key similarities that sodium hydroxide and potassium hydroxide share:
- Appearance: They look similar in solid form (white flakes, pellets, or coarse powder).
- Low flammability: In both solid and liquid form, NaOH and KOH have low flammability properties.
- Corrosive: Both are very corrosive and can cause damage to eyes, skin, mucous membranes, or the respiratory system. When handling, it’s vital to wear the appropriate protective equipment.
- Hygroscopic and deliquescent: Both NaOH and KOH are highly reactive with water and moisture. They require storage in sealed, air-tight containers. They absorb water (hygroscopic), and also dissolve in water (deliquescent).
- Exothermic water reaction: When added to water, a potassium hydroxide and sodium hydroxide solution produce a lot of heat. An aqueous solution made from any of these two compounds must be handled with caution.
- FDA-approved for use in food: Both chemicals are commonly used in various food processing applications as a thickener and stabilizer. NaOH helps to remove skins from fruits and vegetables during canning and create a golden-brown color on pretzels.¹
- Commercial applications: Both chemicals play a key role in the production of various goods, including paper and petrochemical products, alkaline batteries, and various cleaning products (such as drain cleaners) and detergents.¹

Difference between sodium hydroxide and potassium hydroxide
As it pertains to soapmaking, you’ll find a few key differences between NaOH and KOH. The main difference between sodium hydroxide (NaOH) and potassium hydroxide (KOH) for making soap is the type of soap that is produced. Sodium hydroxide produces hard soap, also known as bar soap, while potassium hydroxide produces liquid soap.
This is because potassium hydroxide is a more soluble alkaline compound than sodium hydroxide. You’ll see potassium hydroxide as an ingredient for making homemade liquid soaps, as it produces soap with a softer and more creamy texture than soap made with sodium hydroxide.
On the other hand, sodium hydroxide is often used for making solid bar soaps, as it produces a harder and more durable soap. The choice between the two depends on the type of soap you want to make, along with its finished properties.
On a molecular level, potassium hydroxide is slightly smaller. This means that it’s better able to cut through grease and oil. KOH is also more water-soluble and able to rinse away grease, especially with hot water.²
However, if you’re looking to make your own homemade soaps, most soap makers prefer to use sodium hydroxide for its hardening properties. It’s also slightly less expensive.
If you’re looking to buy a lot of potassium hydroxide, it’ll cost you more—about three times more per ton. However, if you’re just looking to buy a small amount for home-use, the price difference is pretty negligible.²
It costs more to produce the compound potassium chloride. On the other hand, it’s relatively cheaper to make sodium chloride, which is just regular old table salt.
This price difference is a large reason why most manufacturers choose sodium hydroxide for the majority of soapmaking processes today.³
Table: What’s the difference between sodium hydroxide and potassium hydroxide for soapmaking?
| Sodium hydroxide (NaOH) | Potassium hydroxide (KOH) |
|---|---|
| Creates opaque bar soaps | Soaps are typically clear (although certain added elements can make it cloudy) |
| Soaps are harder (the types of bar soap most of us are accustomed to) | Soaps are softer, used for liquid soaps |
| Water-soluble | Greater water solubility, able to rinse away oil |
| Typically, less expensive | More expensive (about 3 times more expensive per ton) |
| Stronger exothermic reaction with water (gives off more heat) | Strong exothermic reaction with water |
| Slightly larger on a molecular level | Smaller on a molecular level: able to better penetrate oil and rinse away |
| Sodium hydroxide solutions leave a yellowish trace | Potassium hydroxide solutions leave a whitish trace |
What the old-timers used to make soap
The art of soapmaking is older than the hills. One of the earliest accounts of soap was in 3000 B.C. by the Sumerians who made a slurry of water and ashes to clean grease from raw wool to prepare fabric to be dyed.⁴
How did settlers and old-timers make their own soap?
They created their own form of potash lye by mixing wood ash (from hardwood trees) and water. They collected fat and drippings from farm animals, combined this with their homemade potash, and boiled it to make their own soft soaps.
Where can you buy sodium hydroxide and potassium hydroxide lye?
You can buy NaOH and KOH at most online soap suppliers such as Amazon, Bulk Apothecary, and Wholesale Supplies Plus. When buying lye for soapmaking, make sure to only buy pure lye without any additives or additional chemicals. Many drain cleaners found in local hardware stores are labelled as lye, but have toxic chemicals that you don’t want anywhere near your skin.
Check out our article on where to buy sodium hydroxide for soapmaking.
The takeaway
While sodium hydroxide and potassium hydroxide are similar in many ways, they can’t be used interchangeably in soapmaking. When deciding which chemical compound to use, it mainly depends on what type of soap you’d like to make. Sodium hydroxide is commonly used today to make soap as it’s less expensive and it creates hard bars of soap we’ve become accustomed to.
When making your own soap at home, ensure you purchase pure, high-quality lye from a reputable supplier for best results. And don’t let your sodium hydroxide sit too long before using it up—it has a shelf life.
Related questions
Can you use both sodium hydroxide and potassium hydroxide in a soap recipe?
Yes, some soapers like to combine the two to create a certain texture in the finished bar of soap (although I’ve never done this yet myself!). You’ll have to run your numbers carefully through a lye calculator as NaOH and KOH have different saponification values.
What is sodium carbonate?
Sodium carbonate is a chemical compound with the formula Na₂CO₃, also known as soda ash or washing soda. It’s a white, odorless powder that is highly soluble in water and has a variety of uses. Sodium carbonate is commonly used in the manufacturing of glass, detergents, and paper. You’ll also find it used as a water softener and as a pH regulator in various industrial processes.
Sodium carbonate is an alkali that’s used for many household cleaning purposes. Don’t confuse this with sodium hydroxide which is much stronger and more corrosive! And, no, you can’t replace sodium hydroxide with sodium carbonate in a soap recipe.
What’s the difference between sodium carbonate and sodium bicarbonate?
Sodium carbonate and sodium bicarbonate are two different chemical alkaline compounds with distinct properties and uses. While both come in white powder form, their main difference lies in their strength. Sodium carbonate (aka washing soda), is a much stronger alkaline substance than sodium bicarbonate (aka baking soda). Washing soda is often used in detergents and as a water softening agent. When using washing soda, take proper precautions to protect eyes and skin.
Sodium bicarbonate, or baking soda is a gentler alkali used in cooking and baking as a leavening agent, as well as an everyday cleaning ingredient and deodorizer.
While sodium hydroxide and potassium hydroxide are similar in many ways, they can’t be used interchangeably in soapmaking. When deciding which chemical compound to use, it mainly depends on what type of soap you’d like to make. Sodium hydroxide is commonly used today to make soap as it’s less expensive and it creates hard bars of soap we’ve become accustomed to.
When making your own soap at home, ensure you purchase pure, high-quality lye from a reputable supplier for best results. And don’t let your sodium hydroxide sit too long before using it up—it has a shelf life.
Related questions
Can you use both sodium hydroxide and potassium hydroxide in a soap recipe?
Yes, some soapers like to combine the two to create a certain texture in the finished bar of soap (although I’ve never done this yet myself!). You’ll have to run your numbers carefully through a lye calculator as NaOH and KOH have different saponification values.
What is sodium carbonate?
Sodium carbonate is a chemical compound with the formula Na₂CO₃, also known as soda ash or washing soda. It’s a white, odorless powder that is highly soluble in water and has a variety of uses. Sodium carbonate is commonly used in the manufacturing of glass, detergents, and paper. You’ll also find it used as a water softener and as a pH regulator in various industrial processes.
Sodium carbonate is an alkali that’s used for many household cleaning purposes. Don’t confuse this with sodium hydroxide which is much stronger and more corrosive! And, no, you can’t replace sodium hydroxide with sodium carbonate in a soap recipe.
What’s the difference between sodium carbonate and sodium bicarbonate?
Sodium carbonate and sodium bicarbonate are two different chemical alkaline compounds with distinct properties and uses. While both come in white powder form, their main difference lies in their strength. Sodium carbonate (aka washing soda), is a much stronger alkaline substance than sodium bicarbonate (aka baking soda). Washing soda is often used in detergents and as a water softening agent. When using washing soda, take proper precautions to protect eyes and skin.
Sodium bicarbonate, or baking soda is a gentler alkali used in cooking and baking as a leavening agent, as well as an everyday cleaning ingredient and deodorizer.
New to making soap? 🧼❓
👉We have a fantastic overview on the whole soapmaking process here: read our Timeless Guide To Soapmaking.
If you would like to see our soapmaking posts organized by topic type, see our Soapmaking Collection.
Would you like more timeless tips via email?
Fun tips to help you live an independent, self-sustaining lifestyle. Opt-out at any time.

References
- Chemical Safety Facts, Sodium Hydroxide, https://www.chemicalsafetyfacts.org/chemicals/sodium-hydroxide/. Accessed April 2023.
- eClean Magazine, Sodium Hydroxide vs. Potassium Hydroxide: What is the difference?, https://www.ecleanmag.com/sodium-hydroxide-vs-potassium-hydroxide-what-is-the-difference/. Accessed April 2023.
- Britannica, Early synthetic detergents, https://www.britannica.com/science/soap/Early-synthetic-detergents. Accessed April 2023.
- OpenLearn, The history of soapmaking, https://www.open.edu/openlearn/history-the-arts/history/history-science-technology-and-medicine/history-science/the-history-soapmaking. Accessed April 2023.

Author: Josh Tesolin
Josh is co-founder of RusticWise. When he’s not tinkering in the garden, or fixing something around the house, you can find him working on a vast array of random side projects.