7 Household Acids for Cleaning and How To Use Them
RusticWise is supported by its readers. When you purchase through links on our site, we may earn an affiliate commission. As an Amazon Associate, we earn from qualifying purchases. Thank You!
On the pH scale, acids are anything below pH 7. We use acids for cleaning to break down mineral deposits, remove rust, and descale hard water stains. Acidic cleaners range from mild (think lemon juice or vinegar) to strong (hydrochloric acid).
Like all types of cleaners, there’s a right way to use them, and a not-so-good way. (I’ll save you the heartache now and tell you not to use an acid cleaner on your natural stone countertops!)
Let’s take a closer look at common household acidic cleaning products and how to use them effectively to get your home sparkling clean.
What exactly are acids for cleaning?
Acids for cleaning, or acid-based cleaners, are a class of cleaning products or ingredients that are great for:
- Removing rust on metal such as aluminum, brass, bronze and copper.
- Descaling hard mineral deposits from kettles and coffee pots.
- Cutting grease (lemon juice).
- Removing hard water deposits on tile and tub.
- Polishing metal faucets on sinks, showers, and tubs.
As mentioned earlier, acid cleaners are those with a pH level below 7.
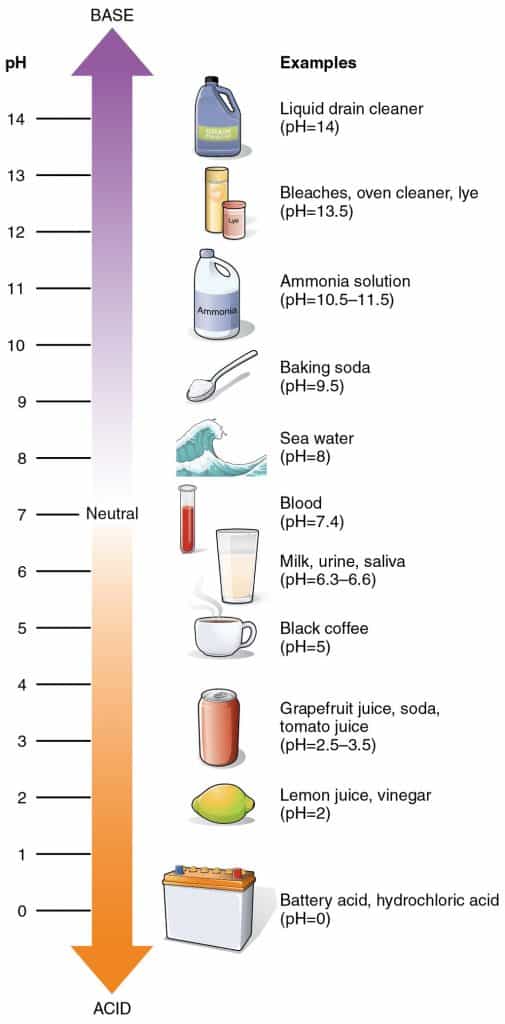
Here’s a quick refresher on the pH scale in case it’s been a while since you last learned about it in science class.
The pH scale runs from 0 to 14, with pH 7 being neutral. Anything below pH 7 is acidic, with the strongest acids having low pH levels. Items above pH 7 are alkaline (or basic).
Keep in mind that a change in number represents a 10-fold change in the alkalinity or acidity of the solution. For example, an acidic cleaning solution with a pH of 2 is ten times more acidic than a cleaner with a pH of 3.¹
Acidic cleaning agents such as lemon juice or vinegar are natural, mild acids with a pH of 2–3. On the other hand, hydrochloric acid has pH 0—it’s as acidic as you can get!
Note: Contrary to popular belief, sodium hydroxide lye is NOT an acid—it’s a strong base, or alkaline. This highly caustic substance plays a key role in soap making, turning fats and oils into soap molecules and glycerin.
Examples of acid cleaners
You probably have some of the following acid-based common cleaning products at home:
- Toilet bowl cleaners
- Tub and tile cleaners
- Vinegar
- Lemon juice
- Mold cleaners
- Metal cleaners
- Rust removers

Credit: Deposit Photos
Safety tips on working with acids for cleaning
I’m sure you already know that acids should be handled with care, but it’s worth repeating, anyway. While mild acids like lemon juice may simply sting any open cuts on your skin (ouch!), use strong acids with extreme caution.
A few examples of strong acids are sulfuric acid and hydrochloric acid, which are found in commercial toilet bowl cleaners.
Always wear protective gloves, avoid getting into eyes, and work in a well-ventilated room to avoid the fumes.
Strong acids can harm skin and eyes, and are poisonous if ingested. Some can also damage clothing, so put on your work clothes. Protect your work area as strong acids can damage some metals, stone, and leather.
Types of acids for cleaning
Weak acids like lemon juice or vinegar are suitable for day-to-day cleaning. You’ll find that these natural and non-toxic acids can be effective cleaners when used properly. You’ll have fewer chemicals in your home and save money on household cleaners!
You’ll find some stronger acids in diluted concentrations in many commercial household products such as toilet cleaners.
Let’s look more closely at common mild acids for cleaning along with a few stronger acids.
Mild acids for cleaning
1. Vinegar (acetic acid)
White vinegar is a mainstay in the world of green DIY cleaning. It gently disinfects, deodorizes, and cleans.
The key to vinegar’s cleaning power lies in its acetic acid content. Most bottles of white distilled vinegar contain around 5 percent acetic acid. Certain pickling vinegars contain more—around 7 percent acetic acid.
Safety tip: Never mix vinegar with bleach as it creates a toxic gas!
Use vinegar to clean around the home in the following ways:
- Remove sticky buildup
- Remove rust from kitchen sinks
- Cut through grease
- Remove tarnish from brass and copper
- Gently lift dirt from hard surfaces
- Descale coffee pots, kettles, and glassware by neutralizing alkaline substances like hard water minerals
- Laundry: soften fabrics and clothing and brighten whites
- To clean and gently disinfect most hard surfaces including glass, tub, and tile
Easy vinegar DIY cleaning solutions
Make your own natural vinegar cleaning solutions:²
- General multi-purpose cleaner: 1 cup vinegar + 1 gallon of water
- Window cleaner: 1/2 cup vinegar + 1 gallon water (2 tablespoons to 1 quart)
What NOT to clean with vinegar
While vinegar is a gentle acid, it’s best to avoid using on stone countertops such as granite or marble as it can etch the surface. Ditto for stone flooring.
Unsealed grout may get damaged. Always do a test patch when using a vinegar solution on any sealed or painted wood surfaces—vinegar may damage the finish.

2. Citric acid and lemon juice
Citric acid derives from lemon juice. It’s a concentrated form of organic acid that’s found in lemons, limes, and pineapples.
Lemon juice helps to deodorize and cut through grease. It has some antibacterial properties and can also help remove stains.
Citric acid has a slight edge over the acetic acid found in vinegar—it acts as a mild reducing agent. This means it can break down proteins in viruses.³
Citric acid is a versatile acidic product. It comes in a concentrated powder form. It’s commonly used in home canning recipes to add a touch of tartness, and as an acidifier.
You can also find citric acid in some eco-friendly cleaners, such as Method All-Purpose Cleaner. It’s important to note that the citric acid in Method cleaner is used as a pH adjuster. The pH level of the Method cleaner is actually alkaline.
You can use lemon juice much the same way as vinegar.
Use citric acid and/or lemon juice to clean around the home in the following ways:
- Remove sticky buildup
- Remove rust from kitchen sinks
- Cut through grease
- Remove tarnish from brass and copper
- Gently lift dirt from hard surfaces
- Descale coffee pots, kettles, and glassware by neutralizing alkaline substances like hard water minerals
- Clean and deodorize cutting boards
- Add a splash of lemon juice to any natural DIY cleaner for a fresh scent
Easy lemon juice DIY cleaning solution
Add a splash of lemon juice to a baking soda paste to create a mild abrasive.
What NOT to clean with lemon juice or citric acid
Go easy on the silverware, or silver-plated items as citric acid may remove some fine details. Porous stone surfaces should also be avoided as well as any painted, or sealed wooden surfaces (some hardwood floors.)⁴
3. Cream of tartar
Contrary to its name, cream of tartar is not actually creamy. It comes in powdered form and is a byproduct of the winemaking process.
While it’s often used in baking (making those meringues light and fluffy), it also is a mild acidic ingredient useful for cleaning.
Cream of tartar is non-abrasive, making it safe for most surfaces. It acts as a brightener and stain remover.
Use cream of tartar to clean in the following ways:
- Brighten aluminum cookware (the bottoms of pans that are burnt and blackened)
- Brighten coffee pots and kettles
- Clean exterior of appliance surfaces such as refrigerators and ovens
- As a stain remover on clothing
How to use cream of tartar to clean
Make a paste:
Sprinkle a bit of cream of tartar into a small bowl. Add just enough water (about 1–2 tablespoons) to create a paste. Apply to surfaces to brighten.
Sprinkle it on:
For stained cookware or scratched dinnerware, simply sprinkle a bit of cream of tartar directly onto the problem area. Add a bit of hot water and let it sit for several minutes. Gently scrub with a non-abrasive sponge or cleaning cloth.

4. Hydrogen peroxide
Hydrogen peroxide is a weak acid that acts as a milder alternative to bleach. It removes stains, kills (some) germs and mold, and gently brightens.⁵
You might have a bottle of it in your medicine cabinet to treat minor wounds (although this practice seems to be falling out of practice in favor of using plain soap and water).
Safety tip: As a strong oxidant, hydrogen peroxide should NOT be mixed with anything other than water. Do NOT mix with ammonia, bleach, or vinegar. ⁵
Use hydrogen peroxide to clean in the following ways:
- Disinfect surfaces
- As a gentler chlorine bleach alternative
- Lift stubborn stains on carpets or clothing
How to use hydrogen peroxide to clean
To wipe down or disinfect surfaces, make a simple solution of 1 part hydrogen peroxide to 2 parts distilled water. Add to a spray bottle and use as needed.
Check out our article on removing carpet stains with household products, including hydrogen peroxide.
What NOT to clean with hydrogen peroxide
Use hydrogen peroxide with caution. It may damage some metals, including aluminum, copper, brass, and zinc. It can also fade some fabrics, so it’s best to do a small patch test first.⁵
Strong acids for cleaning
Sometimes you need to break out the big guns for cleaning stubborn stains. As mentioned earlier, these strong acids can clean off more than you bargained for—use with caution.
All the acids listed below are poisonous—keep away from children and pets.
Wear protective gloves, eyewear, and work in a well-ventilated room when using the following strong acids for cleaning.
Safety precautions: These strong chemicals may react with other metals. When using these strong acids, avoid using two different metals together, which may cause damage. For example, avoid soaking a metal inside another metal container.⁶
5. Oxalic acid
Need to get rid of some tough rust stains? Break out the oxalic acid, one of the strongest organic acids.
This is a common chemical found in many strong household cleaners, such as commercial rust removers and deck cleaners.
Oxalic acid works quickly to remove rust stains and renew wooden decks.
Use oxalic acid to clean in the following ways:
- Remove rust stains on metals, ceramic tile, wall tiles, and carpet
- Restore wooden decks to remove the top soiled layer
- Spruce up brick siding
Safety notes on using oxalic acid
Oxalic acid is a very strong and poisonous chemical. Always wear protective gloves and eyewear, as oxalic acid can harm skin, eyes, and clothing. Follow the directions on your bottle carefully and avoid using on any non-recommended materials.
Once you’ve finished using oxalic acid, throw away any cleaning cloths or brushes that have come in contact with it. (This prevents accidentally transferring the toxic chemical to other frequently used utensils.)⁶
6. Hydrochloric acid (muriatic acid)
A very strong concentrated acid, hydrochloric acid banishes rust and kills bacteria. You’ll often find this harsh chemical diluted forms in heavy-duty toilet cleaners and some exterior cleaning solutions.
Another common name for hydrochloric acid is muriatic acid. Muriatic acid is a term that refers to a hydrochloric acid solution with roughly 30 percent concentration.⁷
This chemical releases toxic fumes, so always work in a well-ventilated area. And don’t forget to put on your protective gear and safety goggles!
Use hydrochloric acid to clean in the following ways:
- Clean heavily soiled toilets
- Clean concrete and bricks
- Remove rust from metals (especially effective against iron and manganese oxides)⁷
- Clean irrigation wells
- Remove scaling buildup
7. Sulfuric acid
This powerful acid is commonly used in heavy-duty drain cleaners to blast away clogs. It should be used as a last resort in cases where all other milder solutions (dish soap, plunging, and using a snake) have failed.
Sulfuric acid is so powerful it may also damage your drains and plumbing. It’s NOT recommended for regular use.
Sulfuric acid like other strong acids is extremely corrosive and releases fumes. Work in a well-ventilated room and wear all protective gear.
Keep sulfuric acid away from certain plastics such as nylon and vinyl.
Other types of cleaning ingredients
For the most effective cleaning, it helps to have a general knowledge of the main types of cleaning ingredients. Besides acid cleaning agents, there are several other major categories of cleaning ingredients:
- Abrasives: Materials or ingredients ideal for scouring off dirt, such as steel wool pads, or plastic scrubbies. It can also refer to abrasive mineral-based ingredients such as baking soda or borax. The term abrasives also includes chemical-based scouring agents.
- Alkalis: Cleaning ingredients with an alkaline solution (anything above pH 7). Alkalis are great for cutting through grease, deodorizing, and cleaning. Examples of alkalis include baking soda, borax, and washing soda. Strong alkalis such as lye are very caustic.
- Bleach and disinfectant: Chlorine bleach is a common and effective disinfectant which contains sodium hypochlorite. Disinfectants kill microorganisms.
- Surfactants: The key components of soaps and detergents. Surfactants are effective at banishing oily or greasy molecules, and removing food residue. Surfactants can be plant-based or petroleum-based.
Related questions
Is muriatic acid a cleaning agent?
Yes, muriatic acid is a type of powerful acidic cleaner that has a solution of hydrochloric acid at roughly 30 percent concentration. Muriatic acid is commonly used for industrial purposes such as cleaning irrigation wells and cleaning concrete.
What are some common household acids and bases?
Here are a few common household acids:
- Lemon juice
- Vinegar
- Tomato juice
- Grapefruit juice
- Toilet cleaners
Here are a few common household bases:
- Baking soda
- Bleach
- Oven cleaner
- Drain cleaner
- Most soaps and detergents
👉 If you like this post, see other Timeless Cleaning Tips You Need To Know. 🌟
Would you like more timeless tips via email?
Fun tips to help you live an independent, self-sustaining lifestyle. Opt-out at any time.

References
- U.S. Department of the Interior, pH Scale, https://www.usgs.gov/media/images/ph-scale-0. Accessed May 2022.
- Utah State University, Home Cleaning Chemicals, https://extension.usu.edu/archive/home-cleaning-chemicals. Accessed May 2022.
- University of Pittsburgh, When Choosing Cleaners it Helps To Know Your Chemistry, https://news.engineering.pitt.edu/when-choosing-cleaners-it-helps-to-know-your-chemistry/. Accessed May 2022.
- University of Arkansas, Clean and Green Homemade Cleaners, https://www.uaex.uada.edu/environment-nature/water/quality/clean-green-homemade-cleaners.aspx. Accessed May 2022.
- University of Colorado Boulder, Common Chemicals Used for Cleaning & Decontamination Guideline, https://www.colorado.edu/ehs/resources/common-chemicals-used-cleaning-decontamination-guideline. Accessed May 2022.
- New Mexico State University, College of Agriculture and Home Economics, Selection and Use of Home Cleaning Products, https://cannon.tennessee.edu/wp-content/uploads/sites/85/2020/04/4H-Judging-CDM-Study-Cleaning-Products.pdf. Accessed May 2022.
- North Dakota State University, Care and Maintenance of Irrigation Wells, https://www.ndsu.edu/agriculture/ag-hub/publications/care-and-maintenance-irrigation-wells. Accessed May 2022.

Author: Theresa Tesolin
Theresa is co-founder of RusticWise. She helps people unleash their inner DIY spirit by encouraging them to get dirty and make or grow something from scratch.