What Are Surfactants in Soap? Synthetic vs. Natural Surfactants
Soap is something we use every day, but how exactly does it work to clean?
The key to an effective clean lies in key ingredients: surfactants (aka surface-active agents). Surfactants in soap help reduce water’s surface tension and allow dirt and oil to easily wash away.
We’ll dive into the soapy mechanics of surfactants, the four main types of surfactants, and the major synthetic surface active agents you’ll find in soap. Plus, we’ll look into natural or naturally-derived surfactants too.
What exactly is a surfactant?
In a nutshell, a surfactant is any substance that reduces surface tension of water (makes it “wetter”).
If you sprinkle a few drops of water onto a plate, the water droplets form beads.
But, if you add soapy water, you’ll notice that the water spreads more evenly across the surface of the plate. That’s the beauty of surfactant molecules!
According to Britannica, “a surfactant, also called surface-active agent, [is a] substance such as a detergent that, when added to a liquid, reduces its surface tension, thereby increasing its spreading and wetting properties.”¹
Besides reducing surface tension, surfactants also act as emulsifiers, or foaming/lathering agents, and dispersants. They can also work to penetrate surfaces for a deeper clean.

The definition of true soap
Now before we dive further into surfactants, we should also define the term “soap” which many people use interchangeably with “detergent.” These two terms have key differences.
The U.S. Food & Drug Administration (FDA) defines real soap as a product that is made mainly of “alkali salts of fatty acids.”
We won’t delve into the science of soap making here, simply put, true soap is made by combining a strong alkaline (typically sodium hydroxide or potassium hydroxide lye) with oils and fats.
Most of the products you’ll find on store shelves (liquid handwash and laundry detergents, for example) are actually synthetic detergents. Most synthetic detergents today derive from petroleum-based products. Occasionally, they also come from oils and fats.
You might have heard of the term “syndet” soap. This stands for synthetic detergent. Sometimes you’ll see products labelled as beauty bars or moisturizing bars, which are not true soaps by definition.
A closer look at 4 types of surfactants in soap
The reason we had to differentiate between syndets and true soap is because surfactants are key ingredients added to detergents. With true soap, soap is a surfactant—in and of itself. Confused? Keep reading!
The four types of surfactants and examples of each
We can group surfactants into four categories based on their ionic (or nonionic nature), as well as their electrical charge.²
1) Anionic surfactants: The primary group of surfactants which provide strong cleaning qualities and also include soap and other syndets. Anionic detergents do the “heavy lifting” so to speak, when cleaning. An anionic-active molecule has a long carbon chain which attaches to a sulfo group (―SO3), giving it a negative charge. Some examples of anionic surfactants include:
- Natural soap
- Linear alkylate sulfonate(one of the most commonly used)
- Alkane sulfonate
- Alkyl ethoxylate sulfate
- Alkyl glyceryl sulfonate
- Alkyl sulfate
- Alpha olefin sulfonate
- Sodium lauryl sulfate (SLS)
- Sodium laureth sulfate (SLES)
2) Nonionic surfactants: These surfactants have a neutral charge and are great at cutting through grease (oily particles). These wetting agents are often used in the final rinse in dishwasher liquids for a more thorough rinse. Examples of nonionic compounds are the group of ethoxylated alcohols.
3) Cationic surfactants: These have a positive charge. Cationic compounds boost disinfecting and deodorizing properties in household products. An example is alkyl dimethyl benzyl ammonium chloride, which belongs to the group of quaternary ammonium compounds. Manufacturers add these to bathroom cleaners, or fabric softeners. They don’t have the highest cleaning power and are often used together with other surface-active agents.
4) Amphoteric surfactants: Also known as ampholytic surfactants, these may act either as anionic or cationic depending on whether the detergent is more acidic or alkaline. Manufacturers often add these to shampoos and conditioners. They’re also found in industrial cleaners. Examples include:
- Alkylamidopropylamine N-oxide (APAO)
- Alkyldimethylamine N-oxide (AO)
- Alkylbetaine (Bt)
- Alkylamidopropylbetaine (APB)
- Cocamidopropyl betaine
- Cocoamphoacetate and cocoamphodiacetate
The takeaway: Natural soap is an anionic surfactant. Detergents contain specially formulated combinations of the above surface-active agents to achieve a desired effect.
What cleaning products or personal care products are surfactants used in?
Almost every household cleaning product or body care product contains some surfactants. Here are just a few examples.
Cleaning products:
- Dishwasher detergent
- Liquid dish soap
- Laundry detergent
- Stain removers
- Bathroom cleaners and disinfectants
- Car wash soap
Personal care products:
- Commercial hand soap (bar and liquid)
- Facial cleansers
- Shampoo and conditioner
- Shower gel and body washes
- Toothpaste

How do surfactants in soap work?
Let’s get into the nitty-gritty science of soap.
You know already that oil and water don’t mix. This is a problem as most things we want to wash away are fat molecules, oily soils, or particulate soils.
This is where surfactants come in handy.
As mentioned earlier, surface-active agents reduce the interfacial tension between water and the surface you’re trying to clean. This allows oils and water to get cozy, instead of repelling one another. This makes for aqueous solutions that actually clean!³
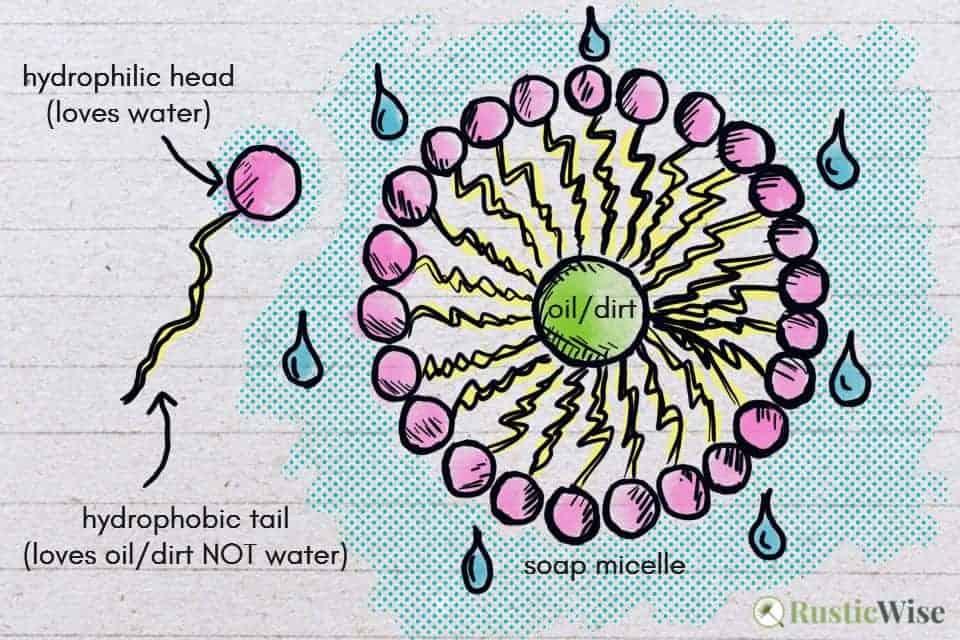
Soaps and detergents work in a similar manner. They have molecules which contain a hydrophilic component (water-loving), and a hydrophobic part (avoids water, but loves grease).
In this case, the soap molecule has a hydrophilic head and a hydrophobic tail.
When you apply soap to a surface, the molecules form soap micelles or clusters which group around oil and dirt particles. Oil and dirt get trapped in the middle of each cluster. The grease-loving tails latch onto the oil or dirt particle. The water-loving heads all point outwards.
This allows oily molecules to easily wash away!
Are soaps surfactants?
This leads us to ask, are natural bars of soap surfactants? Yup. Homemade soap bars are the original man-made surfactant. They mix well in both water and oil.
On a molecular level, fats and oils in soaps are called triglycerides because they contain three fatty acids. These are each attached to an oxygen atom and to glycerol, or glycerin.⁴
Unlike syndet bars, natural soap bars contain glycerin, a skin-nourishing humectant which draws and retains moisture on skin. Glycerin is a natural byproduct of the traditional soap making process.
The difference between soap and detergent is that natural soap does NOT contain added synthetic surfactants. Instead, they work by taking on the properties of the various vegetable oils and/or animal fats used in the soap recipe.
For example, soaps high in coconut oil are very cleansing, and have lots of fluffy lather. Olive oil is very moisturizing on skin, but lower in cleansing and lathering power.
As mentioned above, soap molecules contain a water-loving head and an oil/grease-loving tail. The mechanical rubbing of hands is what actually helps to remove bacteria and viruses from your skin.
So wash like you mean it, getting soap into all nooks and crannies, including under your fingernails! Rub your hands vigorously with soapy water for at least 20 seconds, according to the Centers of Disease Control and Prevention (CDC). You can sing the birthday song twice, to keep track of the time.
What are some natural surfactants for soap?
Two of the most common artificial surfactants found in everyday products are sodium lauryl sulfate (SLS) and sodium laureth sulfate (SLES). Artificial surfactants such as SLS and SLES often get a bad rap for being harsher on the skin. These may leave skin feeling dry or itchy. SLS and SLES derive from petroleum-based products which are not readily biodegradable.
There are other more natural and gentle surfactants that come from natural sources. Two mild surfactants come to mind. They are both made from coconut oil fatty acid and are biodegradable: sodium cocoyl isethionate (SCI) and sodium coco sulfate (SCS). (They look like soap noodles.)
- Sodium cocoyl isethionate (SCI): Also known as baby lather (as it’s gentle enough for a baby’s skin!), SCI is a very mild cleanser that effectively removes oil and dirt but still leaves skin and hair feeling soft. Because it comes from natural origins, it’s commonly used in DIY shampoo bars, liquid soap, whipped soap, shaving cream, facial cleansers and more.
- Sodium coco sulfate (SCS): Slightly stronger than SCI, but still milder than SLS and SLES, SCS is used as a secondary surfactant. It has great foaming properties.
There are also naturally occurring surfactants in the plant world. Two that spring to mind are the soapwort plant and soapberries from the deciduous soapberry tree (belonging to the genus Sapindus).

Credit: Biodiversity Heritage Library / Flickr
These natural surfactants contain saponins which lather and clean:
- Soapwort (Saponaria officinalis): All parts of the soapwort plant contain saponins, but most are concentrated in the roots. You can make your own mild cleanser using dried or fresh soapwort by making a decoction.
- Soap nuts (or soapberries): The dried fruits of the soapberry tree serve as a natural soap. The saponins are concentrated in the shells or husks of the nuts (seeds are discarded). To make your own natural soapy cleanser or soap nut shampoo, simply boil soap nuts in water and strain. Let the liquid cool before applying to hair. Avoid getting in eyes as it stings! I use soap nuts instead of laundry detergent as it’s free of harsh chemical surfactants.
Related questions
Is coconut oil a surfactant?
Technically, pure coconut oil (the stuff you scoop out of a jar for cooking or beauty care) is considered an oil, not a surfactant. Coconut oil is a saturated fat (solid at room temperature) that contains high levels of lauric fatty acid, a type of fat with super cleansing properties.
That’s why many people use it to remove makeup and as a base oil for soap making. However, a coconut fatty acid (which essentially is a coconut soap bar) is a surfactant.
Is liquid Castile soap a surfactant?
Yes, Castile soap is a surfactant. It meets the definition of “surfactant” by reducing surface tension and having both water-loving and oil-loving ends. Castile soap is a natural and biodegradable soap that comes in liquid and solid form. It was once derived from pure olive oil, but now, many formulations also include other plant-based oils such as coconut oil or palm oil.
If you’re making your own DIY products, Castile soap may not be a good substitute for a surfactant, depending on your recipe. The result could be a weaker formulation.
What is the difference between surfactants and detergents?
A surfactant is any compound which reduces the surface tension of water and enables oil and grease to more easily wash away. A detergent on the other hand, is made of various surfactants plus other chemical compounds to produce a finished product with cleansing properties.
In other words, surfactants are one of the main ingredients in synthetic detergents.
New to making soap? 🧼❓
👉We have a fantastic overview on the whole soapmaking process here: read our Timeless Guide To Soapmaking.
If you would like to see our soapmaking posts organized by topic type, see our Soapmaking Collection.

References
- Britannica, Surfactant, https://www.britannica.com/science/surfactant. Accessed April 2022.
- American Cleaning Institute, Ingredient Glossary, https://www.cleaninginstitute.org/understanding-products/ingredients/ingredient-glossary. Accessed April 2022.
- University of Illinois at Urbana-Champaign, Q & A: Soap!, https://van.physics.illinois.edu/qa/listing.php?id=465&t=soap!. Accessed April 2022.
- Britannica, Soap, https://www.britannica.com/science/soap. Accessed April 2022.

Author: Josh Tesolin
Josh is co-founder of RusticWise. When he’s not tinkering in the garden, or fixing something around the house, you can find him working on a vast array of random side projects.