The pH of Dish Soap: How It Affects Cleaning Power and Your Skin
If you’re looking for ways to get a better clean around your home, understanding the pH of common household products is a great place to start. Simply put, the pH scale shows how acidic or alkaline (basic) a substance is.
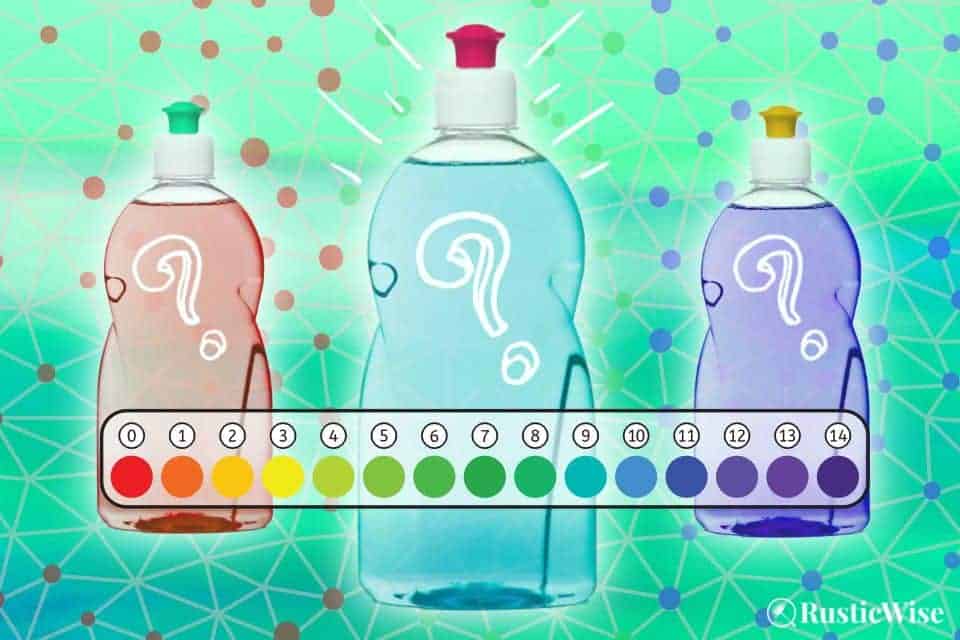
Liquid dish soap is one of the most common cleaners we all have at home. The pH of most dish soap ranges from pH 8 to 10, depending on the brand, with a few having a pH between 7 and 8. This makes most liquid handwashing dish soaps neutral to alkaline.
Dish soap is a type of (mostly) alkaline surfactant that cuts through grease and removes oily food particles and grime. More acidic cleaners such as vinegar or lemon juice are better at removing hard minerals, soap scum, and rust.
We’ll take a closer look at the pH of dish soap, other common household cleaners, and how you can harness their pH levels to clean more effectively.
A quick refresher on the pH scale
In case it’s been awhile since you last learned about the pH scale in science class, here’s a quick refresher for you.
The term “pH” stands for power of hydrogen. It indicates how acidic or basic an aqueous solution is.
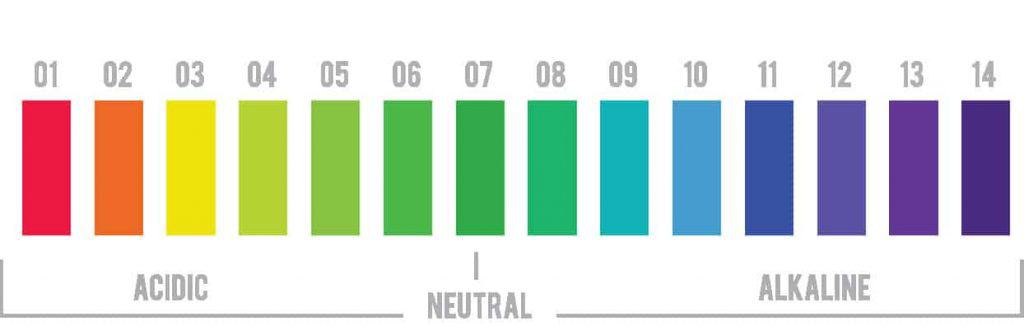
The pH scale ranges from 0 to 14. A pH of 7 is neutral. Anything below 7 is acidic, and anything above pH 7 is alkaline or basic.
It’s important to remember that a change in number represents a 10-fold change in the alkalinity or acidity of the solution. For example, a dish soap with a pH of 9 is ten times more alkaline than dish soap with a pH of 8.¹
Why pH matters in cleaning: acids vs. alkalis
It’s important to understand the difference between acidic cleaning products vs. alkaline substances. Let’s take a closer look.
How to use acids in cleaning
Cleaning products that are acidic are great for removing hard water stains (think mineral deposits) and rust stains. Some metals that have tarnished, including aluminum, brass, bronze, and copper, may benefit from an acidic treatment.²
Some acids can both clean and disinfect at the same time.
Acids range in strength from mild to strong. The stronger the acid, the more likely it is to irritate eyes and skin. For example, some commercial toilet cleaners are most often highly acidic (to dissolve limescale), so it’s best to protect yourself by wearing gloves and splashes.
How to use alkalis in cleaning
Alkaline products include soaps and detergents, all-purpose cleaners, and baking soda. Alkalis are great for most everyday cleaning purposes—removing dirt or other organic matter, cutting through grease, and cleaning oily spills.²
Alkaline products range from mild soap (such as liquid dish soap) to very strong and caustic substances such as lye.
Now that you’re armed with a basic knowledge of pH and how it works, you can tailor your cleaning products according to your needs!

What’s the pH of dish soap?
The pH of dish soap varies widely, but most products fall between pH 8 and 10. (There are a few with a pH between 7 and 8). This makes most dish soaps a neutral to mild alkaline solution perfect for tackling a pile of dirty dishes.
While it does a great job of getting rid of stuck-on food particles and grease from last night’s casserole dish, liquid dish soap is also a versatile mild soap for everyday cleaning around the house.
Table of pH levels of common dish soaps
| Dish Soap | pH level* |
|---|---|
| Dr. Bronner’s Peppermint Pure-Castile Liquid Soap | pH 9–10 |
| Dawn Ultra Dishwashing Liquid, Original Scent | pH 9.0–9.2 |
| Ivory Dishwashing Liquid | pH 9 |
| Joy Ultra Dishwashing Liquid (Lemon) | pH 9 |
| Seventh Generation Purely Clean Dish Liquid & Hand Wash | pH 7.9–8.1 |
| Mrs. Meyer’s Clean Day Dish Soap – All Fragrances | pH 7.4–7.9 |
| Palmolive Dishwash Hand Liquid Original | pH 7.2 |
Note that liquid Castile soaps are concentrated formulas that should be diluted with water first before using. Other liquid dish soaps don’t require dilution. So a diluted Castile soap solution would have a lower alkaline pH level, bringing it closer to a pH neutrality.
And just for comparison, a bar of natural homemade soap typically has a pH between 8 and 10 making it alkaline.
How the pH of dish soap affects cleaning power
Most people use dish soap when there is oil or grease to banish, or organic particles to remove (stuck-on food, or dirt, for example). Dish soap is a mild alkaline solution designed for removing fat, oil, and grease effectively.
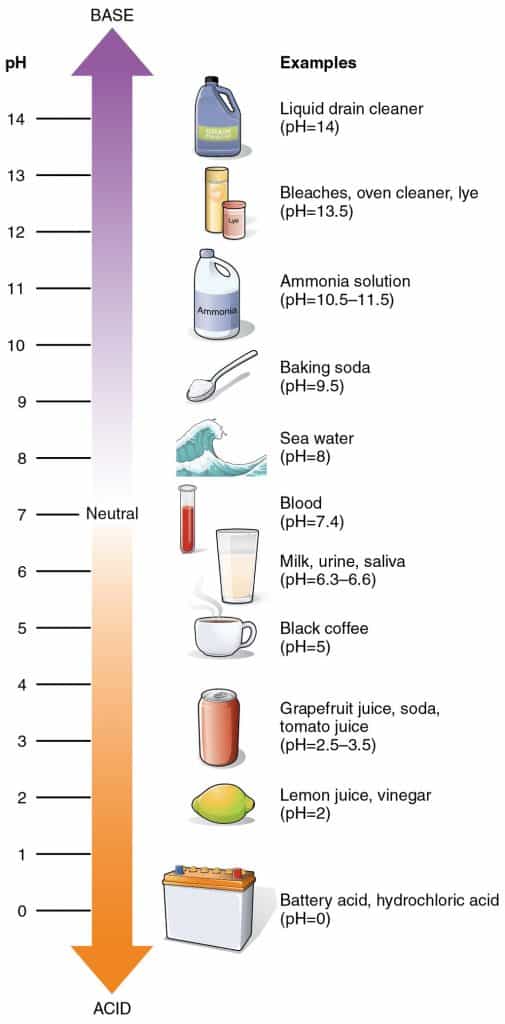
If you recall from the graphic above, a few examples of strong alkaline solutions are drain cleaners, bleach, ammonia, and oven cleaners.
Handle strong alkalis with care. Remember to wear protective gloves, eyewear, and long sleeves to prevent skin contact. You only break out the big guns when there’s some serious cleaning to do.
So, what about those times when you want a stronger dish soap to clear up a clogged kitchen sink, or unclog a backed up toilet? Reach for a more alkaline dish solution, or one with a higher pH level.
From the chart above, you can see that Dawn dish soap is more alkaline than other brands such as Palmolive. Remember that each number change on the pH scale represents a 10-fold change.
So, if Dawn has a rough pH of 9 and Palmolive has a rough pH of 7, that means that Dawn is about 20 times more alkaline!
The more alkaline something is, the better it’s able to degrease and unclog!
There’s a reason so many plumbers swear by Dawn dish soap as a DIY remedy to clear up clogged kitchen sinks. It’s much stronger than other leading brands for removing fat, oil, and grease—the prime culprits of drain clogs. (And no, I’m not being paid by Dawn for saying this.)
A closer look at dish soap and how it cleans
Did you know commercial “soaps” are not true soaps by definition, but actually detergents. Detergents are chemical-based cleaners typically derived from petroleum and fatty acids.
These liquid dish detergents remove food residue, create plenty of bubbles and lather, rinse clean (without leaving spots or film), AND be mild enough for hands.
Dish soap contains surfactants, which stands for surface active agents. Surfactants are simply compounds that reduce surface tension of water and enable water to spread more evenly across surfaces for better cleaning. This allows oil and dirt to more easily wash away.
Soap molecules have a water-loving (hydrophilic) head, and a grease-loving (hydrophobic) tail.
When mixed with water, clusters of soap form, which are called soap micelles. Each micelle surrounds oil or dirt particles in a circle. The grease-loving tails latch onto the oily molecules, while the water-loving heads face outwards.
This allows oily molecules and leftover food to easily wash away!

What’s in dish soap?
Dishwashing detergent for handwashing dishes is considered a mild, or light-duty cleaning solution. Here are the main ingredients you’ll find in most regular dish soap:³
- Surfactants (mostly anionic surfactants which remove grease to get dishes clean) including sodium lauryl sulfate (SLS) and sodium laureth sulfate (SLES)
- Ethyl alcohol (used to control viscosity and acts as a solvent)
- Suds and lather boosters
- Stabilizers to ensure a long shelf life (this includes acyl or fatty acid ethanolamides)
- Colorants or opacifying agents
- Fragrances
Is dish soap safe for hands?
Dish soap designed for handwashing dinnerware is fairly mild on skin and hands (although wearing a pair of gloves certainly doesn’t hurt!).
However, some of the key ingredients may prove irritating to those with sensitive skin or dry skin. Most commercial liquid detergents contain sulfates such as sodium lauryl sulfate (SLS) which is a known skin irritant.
Other harsh chemicals are found in the artificial fragrances and colorants.
The pH of healthy skin ranges from pH 5.4–5.9. This makes skin naturally acidic.⁴ This acidity maintains a healthy bacterial flora to flourish on skin, forming a protective layer.
Using a more alkaline product such as dish soap may temporarily alter the skin’s natural acidity levels, potentially causing dry or itchy skin. (You can check out our article about the pH of hand soap here for more details.)
If you’re wanting a mild dish soap that’s gentler on skin, choose one of the more pH neutral soaps such as Palmolive. Alternatively, you could put on a pair of dish gloves. Or, use more natural dish soaps without harsh chemicals or artificial fragrances, such as making your own DIY liquid Castile soap.
The takeaway: While pH plays a role in skin reaction and soap, it isn’t everything. The ingredients found in common cleaning products such as dyes and synthetic fragrances all play a role in how your skin reacts to it.
Other uses of dish soap (besides handwashing dishes)
Liquid dish soap when mixed with a bit of warm water is a cleaning workhorse! Here are just a few ways to use it to clean around the house (besides washing dishes, of course!).
- Unclog a kitchen sink: Dawn dish soap paired with a hot water rinse often helps clear most fat, oil, and grease from kitchen drains.
- Clear a clogged toilet: Stubborn clogs in a toilet trap may loosen with a dish soap treatment.
- Clean jewelry: Silver, gold, or platinum jewelry will look sparkly again with a soapy dish soap soak.
- Clean walls: Clear off those fingerprints and wash walls with a bit of dish soap.
Check out more ideas on how to use dish soap and other mild soaps for cleaning.
Related questions
Is dishwashing soap pH neutral?
Most commercial dish detergents are alkaline (or basic) with a pH ranging between 8 and 10. But, there are some types of dish soap that are considered neutral detergents such as Palmolive (pH 7.2), and some Mrs. Meyer’s dish soap (pH 7.4–7.9).
What is the pH of drain cleaner?
Most drain cleaners have a pH of 14, making it extremely alkaline and caustic. (Remember that the pH scale runs from 0 to 14, so drain cleaner is as alkaline as you can get!)
When using drain cleaners, it’s important to protect your eyes and skin, and avoid inhaling the fumes. Before resorting to chemical drain cleaners, try a few gentler sink clearing hacks first, such as using dish soap or vinegar.
What is the pH of vinegar?
Vinegar has a pH of 2, making it acidic. Acidic cleaning ingredients are great for removing hard water deposits and rust stains. Avoid using vinegar on stone countertops or other stone surfaces, or hardwood flooring. Vinegar may also damage some rubber components if used in dishwashers, for example.
👉 If you like this post, see other Timeless Cleaning Tips You Need To Know. 🌟
Would you like more timeless tips via email?
Fun tips to help you live an independent, self-sustaining lifestyle. Opt-out at any time.

References
- U.S. Department of the Interior, pH Scale, https://www.usgs.gov/media/images/ph-scale-0. Accessed May 2022.
- New Mexico State University, Selection and Use of Home Cleaning Products, https://pubs.nmsu.edu/_g/G304/. Accessed May 2022.
- American Cleaning Institute, Ingredient Glossary, https://www.cleaninginstitute.org/understanding-products/ingredients/ingredient-glossary. Accessed May 2022.
- Tarun, Jose et al. “Evaluation of pH of Bathing Soaps and Shampoos for Skin and Hair Care.” Indian journal of dermatology vol. 59,5 (2014): 442-4. doi:10.4103/0019-5154.139861

Author: Theresa Tesolin
Theresa is co-founder of RusticWise. She helps people unleash their inner DIY spirit by encouraging them to get dirty and make or grow something from scratch.