What’s the pH of Hand Soap + 5 Ways To Test Your Homemade Soap
The pH of hand soap varies depending on the type of soap, but most homemade soap bars fall between pH 8 and 10 (occasionally higher). This makes handmade soaps alkaline. Commercially produced liquid soaps have synthetic detergents which lower the pH level, making them more acidic, with a pH level around 5.
As we’re all washing our hands more often nowadays, you may experience dry or itchy skin. You may wonder how the pH of hand soap affects your skin, if at all. Let’s look at what different pH levels in hand soap mean, and how to test your homemade soap batch.
Is hand soap an acid or base?
This depends on the type of soap you’re using. All handmade soaps made from scratch with lye are alkaline (at least they should be if everything is done properly!). This is because the traditional definition of handmade soap is, “an alkaline salt of fatty acids.”
Many commercially made liquid soaps lean more towards the acidic side as they use artificial surfactants and emulsifiers that help lower the pH.
pH basics
The pH scale ranges from 0 to 14 where 7 is neutral. Values below pH 7 are acidic and values greater than pH 7 are alkaline, also called basic.
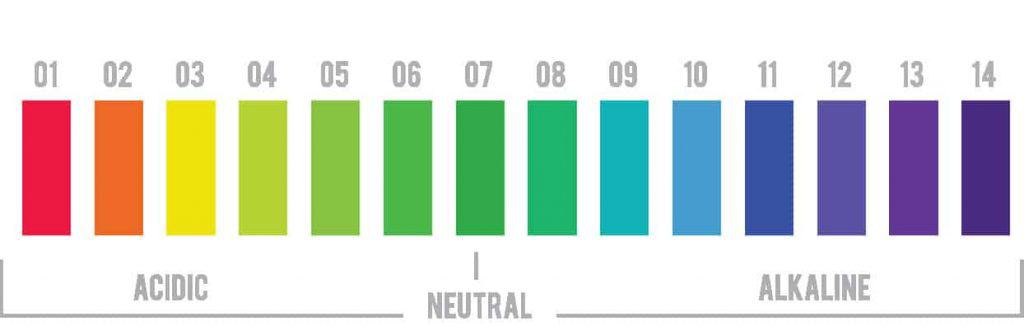
First things first: pH stands for potential of hydrogen in a solution. This is just a measure of the number of free hydrogen vs. free hydroxyl ions in water:
- A solution with greater free hydrogen ions makes something acidic; and,
- A solution with greater free hydroxyl ions is alkaline.¹
On opposite ends of the spectrum, we have extremely acidic or basic elements. For example, battery acid has a pH of 0. Sodium hydroxide lye has a pH of around 13.5.
Here are a few other common household ingredients and their pH:
- Lye: pH 13.5
- Amonia solution: pH 10.5–11.5
- Baking soda: pH 9.5
- Bar hand soaps: pH 8–10, on average
- Blood: pH 7.4
- Distilled water: pH 6.9 (While most people would assume that distilled water should be pH neutral, when exposed to air, carbon dioxide reacts to form a diluted solution of carbonic acid)²
- Milk, urine and saliva: pH 6.3–6.6
- Commercial liquid hand soaps: pH 5–7, on average
- Black coffee: pH 5
- Grapefruit juice, soda pop, and tomato juice: pH 2.5–3.5
- Lemon juice and vinegar: pH 2
- Battery acid: pH 0
It’s important to note that chemicals in water can alter a pH reading.
Each number on the pH scale shows a ten-fold change. For example, a solution with a pH of 2 is 10 times more acidic than a pH of 3.
What is the acid mantle?
The term acid mantle simply refers to skin’s acidic barrier that protects the body from harmful bacteria and contaminants while locking in natural oils and moisture.
The pH of healthy skin ranges between pH 5.4–5.9. As you can see, our skin is naturally slightly acidic, which enables a protective layer of bacterial flora to flourish.³
Using skin products with a higher pH also bumps up the skin’s pH. This causes dryness, sensitivity, and may temporarily alter the natural bacterial flora.
The good news is that the change in the skin’s pH appears to be temporary. A 2015 study⁴ studied the effects of using a soap-based cleanser vs. a mildly acidic cleanser on the forearms of participants over 5 years.
Researchers then measured the pH on the inner forearm of subjects before and after six hours of using a soap bar. They did not notice any difference between the pH levels on skin between the two groups before cleaning, after cleansing, or in the six hours following the cleansing.

A closer look at the pH of hand soap
Here are the pH levels of various types of hand soap according to their MSDS. I’ve included a dish soap as this often performs double duty as both dish and hand soap.
Table: pH of Hand Soaps
| Soap | pH Level |
|---|---|
| Dr. Bronner’s Peppermint Pure-Castile Liquid Soap | 9–10 |
| Dawn Ultra Dishwashing Liquid, Original Scent | 9.0–9.2 |
| Homemade hand soap | 8–10 |
| Ivory Original Liquid Hand Soap | 5.8–7.3 |
| Dove Beauty Bar | 7 |
| Method Foaming Hand Wash – All Fragrances | 5.0–6.5 |
| Mrs. Meyer’s Clean Day Hand Soap Lemon Verbena | 5.0–5.5 |
| J.R. Watkins Foaming Hand Soap | 5.1 |
| Softsoap Antibacterial Liquid Hand Soap | 4.9 |
Why you should test the pH of homemade soap
If you’re a DIY soaper making cold process or hot process soap, you’ll want to ensure it’s not lye heavy and is safe for skin. Testing bar soap pH is important for safety.
For homemade soap, an acceptable range is between pH 8 and 10. (Technically, a range of pH 7 and 10 is acceptable, but it’s very difficult to create a bar of pH neutral homemade soap.)
This is because homemade soap, a fatty acid of an alkali salt, is the byproduct of combining natural oils and lye.
Soaps with a pH greater than 10 may cause skin irritation, or in worst case scenarios, burn the skin.
Timing is key when testing. As cold process soap is still undergoing saponification in the first one or two days, taking a pH reading too early will produce inaccurate results. It’s best to wait for the soap to fully cure before testing (between 4–6 weeks) as the pH may still drop during this time.⁵
5 ways to test pH of handmade (or store-bought)
There are several ways to test the pH level of homemade soaps. (You can also do these tests on store-bought soaps for kicks).
Some methods are more cumbersome than others, and it’s difficult to get an exact reading for most methods.
Note: The following pH tests work with distilled water only. Regular water has impurities, which may alter the test results.
1. pH paper (or litmus paper)
Litmus strip tests are meant for use in a solution. This doesn’t make it easy to test on bar soap.
Many people misuse these papers by placing a drop of distilled water onto a soap bar. They rub the litmus strip onto the wet soap to compare color onto a color-coded diagram on the package.
This may produce inaccurate results. It’s best if you take a chunk of bar soap and create a solution with distilled water. However, the concentration of the solution is the tricky part.
It’s often hard to distinguish between different colors on the box, it provides mixed results.
If you really want to give this method a go, you can buy litmus kits online, or check your local garden center.
2. Digital pH meter
An electronic pH indicator measures the pH of liquid solutions. You can quickly get a pH reading by inserting a probe into a solution. A glass electrode attached to the probe is hooked up to an electronic device that accurately reads pH levels (with decimal points).⁶
While this works great for aqueous solutions, most homemade soap is not liquid.
Digital pH meters can be pricey and also require maintenance (of a buffer solution) and recalibration.
3. Phenolphthalein
Science-y types will be familiar with phenolphthalein. It’s a type of liquid pH indicator that provides a reading from no color to pink.
But once again, phenolphthalein works best when used in a solution. So, if you place a few drops directly onto bar soap to test for neutrality, it might not always work as expected.
Many people use phenolphthalein for hot process soap making to check for doneness.
If it’s pink, it’s alkaline, if it’s clear (colorless) it’s neutral.
While fairly easy to use, having black and white (or in this case, pink and white) results doesn’t exactly provide for nuance.

Credit: Yay Images
4. Purple (red) cabbage juice
Nature is a wonderful thing, and a special compound in cabbage called anthocyanin turns color when it’s mixed with either an acid or a base.⁷
Place a few drops of cabbage juice onto soap. If it turns blue-green, the soap is alkaline or basic (safe to use). If it turns green, it indicates the presence of unsaponified lye. Cabbage juice turns red in the presence of an acid.
To make your own cabbage juice, you’ll need to chop lettuce into small pieces. Blend well and add a cup of distilled water. Strain to remove the cabbage chunks. Store the cabbage juice in a glass jar in the fridge.
Alternately, if you don’t have a blender, you can boil cabbage chunks with a cup of distilled water. Boil for several minutes until water turns red. Let cool and strain.
Store your big batch in a glass bottle or jar and use a small eye dropper to extract a tiny amount.
The cabbage juice method works for all types of handmade soaps: cold process, hot process, and DIY liquid soaps.
5. Old-school tongue test
This is not exactly a method you want to do for your first batch of soap, nor is it a method to use if you’re selling your soap!
The old school method involves sticking your tongue onto a bar of soap (preferably part of the interior section). If you feel a “zap” or a “zing” you’ll know there’s still active lye in the soap.⁵
Tip: No method of testing soap pH is perfect. There are simple signs you can look for to check if your soap batch is lye heavy. Crumbly soap (soap that easily falls to pieces) is a classic sign of too much lye. Sometimes you may see tunneling (or separation of oils) in finished soap. This means the soap did not fully saponify and certain parts may contain active lye.
Is handmade soap better for your skin?
Now that we know that skin is naturally acidic and homemade bars of soap are alkaline, is it better to use store-bought soap that’s acidic?
The topic of handmade soap with natural ingredients vs. commercial soap is hotly debated.
On one side, you can buy syndets or synthetic detergents that are formulated to be more acidic, and therefore supposedly less disruptive to the skin’s acid mantle.
But, most synthetic soaps and cleansers contain chemical surfactants such as sulphates that actually strip away skin’s natural moisture. Artificial fragrances or perfumes used in store-bought soaps can also irritate the skin.
On the other hand, you have a bar of alkaline natural soap made with nourishing oils. We prefer natural homemade soap for a few reasons:
- Quality control: If you’re making the soap yourself, you can control exactly what’s put in it. You can select the exact oils that are suited to your skin.
- The traditional way: People have been crafting soap using basic ingredients (lye + fat + distilled water) for hundreds of years. This is the simplest way to cleanse your skin without harsh chemicals and something we’ve been doing for generations.
- Saves money: If you stock up on a few of your favorite soap making oils in bulk and make large batches of soap, this is a much more budget-friendly way to keep yourself (and your family) clean.
To make an informed decision, check out the ingredients on homemade and store bought soaps. If you have sensitive skin, stick with unscented and color-free soap bars.
👉 For more information, check out our article about homemade vs. manufactured soaps.
New to making soap? 🧼❓
👉We have a fantastic overview on the whole soapmaking process here: read our Timeless Guide To Soapmaking.
If you would like to see our soapmaking posts organized by topic type, see our Soapmaking Collection.
Would you like more timeless tips via email?
Fun tips to help you live an independent, self-sustaining lifestyle. Opt-out at any time.

References
- U.S. Geological Survey, pH Scale, https://www.usgs.gov/media/images/ph-scale-0. Accessed December 2021.
- ReAgent, What is the pH of Distilled Water?, https://www.chemicals.co.uk/blog/ph-of-distilled-water. Accessed December 2021.
- Tarun, Jose et al. “Evaluation of pH of Bathing Soaps and Shampoos for Skin and Hair Care.” Indian journal of dermatology vol. 59,5 (2014): 442-4. doi:10.4103/0019-5154.139861
- Takagi, Y et al. “The long-term use of soap does not affect the pH-maintenance mechanism of human skin.” Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI) vol. 21,2 (2015): 144-8. doi:10.1111/srt.12170
- Crafter’s Choice, Simple Secrets: Testing Soap’s pH Level, https://www.crafters-choice.com/handmade101/learn-to-make-articles/simple-secrets-testing-soaps-ph-level.aspx. Accessed December 2021.
- WiseGeek, What Is a Digital pH Meter?, https://www.wise-geek.com/what-is-a-digital-ph-meter.htm. Accessed December 2021.
- Connecticut Science Center, Science At Play: Red Cabbage Juice Indicator, https://ctsciencecenter.org/blog/science-at-play-red-cabbage-juice-indicator/. Accessed December 2021.

Author: Theresa Tesolin
Theresa is co-founder of RusticWise. She helps people unleash their inner DIY spirit by encouraging them to get dirty and make or grow something from scratch.