Lauric Acid in Soap: Add Cleaning Power + Lather With This Fatty Acid
Every oil, fat, or butter used in soap making has a unique fatty acid profile. Lauric acid in soap is a cleaning powerhouse—when used at very high ratios, it can over cleanse the skin, leaving it dry. Soap making oils high in lauric acid add hardness and a good fluffy lather to soap bars.
The unique fatty acid profile of the oils you use in your batch of soap affects the finished soap. Lauric acids are just one of eight main fatty acids present in fatty acid soaps made from scratch with sodium hydroxide lye.
Let’s take a closer look at lauric acid, how it behaves in soap, and some soaping oils that contain a large amount of this fatty acid.
Fatty acids 101
Fatty acids in soap are the building blocks of handmade soap. Each oil, fat, or butter has a unique fatty acid profile. They determine the qualities that shine through in the finished soap.
Depending on the fatty acid content, you may get a bar of soap that’s hard with fluffy bubbles, or one that’s softer with creamy lather, for example. Most soap makers use a blend of vegetable oils to make an ideal bar of soap: one that’s not too hard, not too soft, with both cleansing and moisturizing properties.
Understanding the properties of fatty acids, such as lauric acid in soap, will help you get better at the craft of soap making.
Fatty acids fall under one of two categories: saturated or unsaturated.
Saturated fatty acids
Most saturated oils or fats are solid at room temperature (think coconut oil and palm oil). Saturated oils make harder bars of soap because their molecular structures allow them to fit together neatly.
Soaps that have a high saturated fatty acid composition are harder, effective cleansers, and typically provide strong lather.¹
There are four types of saturated fatty acids commonly found in soap:
- Lauric acid
- Myristic acid
- Palmitic acid
- Stearic acid
Unsaturated fatty acids
Most unsaturated fatty acids are liquid at room temperature. Examples include avocado oil, olive oil, and safflower oil.
Soaps that have a high unsaturated fatty acid composition are softer, more moisturizing (and less cleansing). They also produce a milder lather that’s gentle on the skin.
The four main types of unsaturated fatty acids are:
- Ricinoleic acid
- Oleic acid
- Linoleic acid
- Linolenic acid
👉 Check out our article for more details about the fatty acids in vegetable oils.
What’s lauric acid?
Lauric acid is a saturated fatty acid with 12 single chain carbon atoms.²
It looks something like this: CH3−CH2−CH2−CH2−CH2−CH2−CH2−CH2−CH2−CH2−CH2−COOH
You’ll find lauric acid esters (which are mostly triglycerides) in vegetable-based fats. At room temperature, lauric acid is a white solid, commonly available as crystals or powder.
Lauric acid has a melting point of 111–115 degrees Fahrenheit (44–46 degrees Celsius).³
Fats derived from the fruits or seeds of trees belonging to the palm family contain ample amounts of lauric acid.
Examples of oils containing plenty of lauric acid include coconut oil, babassu oil, and palm kernel oil. Coconut milk and laurel oil also contain lauric acid.
It’s important to note that PKO contains high lauric acid, while palm oil does NOT contain any. As the name suggests, PKO is derived from the pressed kernels, while palm oil is made of the pressed palm fruit.
If you want to get science-y, the empirical formula for lauric acid is: C₁₂H₂₄O₂
Here’s the chemical structure of lauric acid:
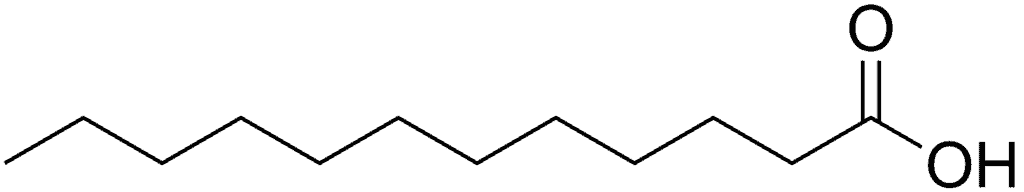
Credit: Wiki Commons
A study examining the role of lauric acid in coconut oil found that, “Lauric acid and monolaurin have demonstrably significant antimicrobial activity against gram positive bacteria and a number of fungi and viruses.”⁴
What does lauric acid in soap do?
If you’re looking to make a hard bar of soap that’s also highly cleansing and creates great bubbly lather, lauric acid is your friend.
The downside to lauric acid in soap is when used in higher ratios, it may strip the skin of natural healthy oils and leave it feeling dry.
To keep the favorable effects of lauric acid, aim to keep the amount of lauric acids between 20 and 30 percent in most soap recipes. Balance the properties of lauric acid by using soft oils with moisturizing qualities such as avocado oil, olive oil, safflower oil, soybean oil, or sunflower oil.
This is just a general guideline, though. You’ll find you can bend or break the rules once you get more familiar with the properties of various oils and fats. For example, you can make a 100 percent coconut oil soap bar that’s not overly drying by bumping up the superfat.

Credit: Yay Images
Soap making oils high in lauric acid
If you want to increase the level of lauric acid in your soap batch, consider adding one of the following oils.
Table: A Comparison of Coconut, PKO, and Babassu Oils High in Lauric Acid
| Coconut Oil, 76 degree | Palm Kernel Oil | Babassu Oil | |
|---|---|---|---|
| Hardness | 79 | 75 | 85 |
| Cleansing | 67 | 65 | 70 |
| Conditioning | 10 | 18 | 10 |
| Bubbly lather | 67 | 65 | 70 |
| Creamy lather | 12 | 10 | 15 |
| Lauric acid | 48 | 49 | 50 |
| Myristic acid | 19 | 16 | 20 |
| Palmitic acid | 9 | 8 | 11 |
| Stearic acid | 3 | 2 | 4 |
| Ricinoleic acid | 0 | 0 | 0 |
| Oleic acid | 8 | 15 | 10 |
| Linoleic acid | 2 | 3 | 0 |
| Linolenic acid | 0 | 0 | 0 |
| NaOH SAP Value | 0.183 | 0.176 | 0.175 |
| KOH SAP Value | 0.257 | 0.247 | 0.245 |
Related questions
What does myristic acid do in soap?
Myristic acid triglycerides are found in plants and animals. It’s most notably found in nutmeg butter, coconut oil, and mammalian milk like that of cows or goats. It’s a saturated fatty acid that’s considered the “softest” of the saturated fats.¹
Similar to lauric acid, myristic acid adds hardness and a strong lather. It too, can be harsh if used in high ratios. Some oils that contain myristic acid are babassu oil, coconut oil, and palm kernel oil.
What does high oleic mean in vegetable oils?
Oils that are labelled as high oleic contain more oleic fatty acids than their “regular” counterparts, which contain more linoleic acids. Vegetable oils that are “high oleic” have a longer shelf life and a higher smoke point (which is better when cooking with it at higher heat).
Most soap makers prefer high oleic safflower oil, or high oleic sunflower oil as they don’t go rancid as quickly. This helps you avoid those awful dreaded orange spots (DOS).
New to making soap? 🧼❓
👉We have a fantastic overview on the whole soapmaking process here: read our Timeless Guide To Soapmaking.
If you would like to see our soapmaking posts organized by topic type, see our Soapmaking Collection.

References
- Grosso, Alicia (2016). DIY Artisanal Soaps: Make Your Own Custom Handcrafted Soaps! Adams Media. pp. 179-180. ISBN 978-1-4405-9408-3.
- Britannica, Chemical composition of fats, https://www.britannica.com/topic/fat/Chemical-composition-of-fats#ref45159. Accessed December 2021.
- American Chemical Society (ACS), Lauric acid, https://www.acs.org/content/acs/en/molecule-of-the-week/archive/l/lauric-acid-myristic-acid.html. Accessed December 2021.
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J Am Oil Chem Soc 92, 1–15 (2015). https://doi.org/10.1007/s11746-014-2562-7. Accessed December 2021. https://link.springer.com/article/10.1007/s11746-014-2562-7

Author: Josh Tesolin
Josh is co-founder of RusticWise. When he’s not tinkering in the garden, or fixing something around the house, you can find him working on a vast array of random side projects.