Citric Acid in Soap Making: The Key To Banishing Soap Scum
Using citric acid in soap making is a useful trick for soap makers who are looking to banish soap scum and prevent oxidation in oils to extend shelf life. It comes in a concentrated powder form. Use between 1 to 3 percent of citric acid of your total oil weight in a soap recipe. (Some recipes for shampoo bars may use a bit more.)
If you have hard water and are tired of scrubbing off soap scum, then adding these tiny white crystals to your next batch of soap may be just the thing you’re looking for!
Using citric acid in soap requires a bit of math as it consumes or neutralizes some of the lye. So break out your calculator and ensure you adjust for the lye lost. Otherwise, you’ll end up with a highly superfatted soap, which will leave you feeling greasy.
We’ll go over the ins and outs of what exactly citric acid does in soap, and how to use it.
Let’s get started!
What exactly is citric acid?
Citric acid is a naturally occurring compound that’s found in many fruits and vegetables, including lemons, limes, broccoli, tomatoes, and some types of peppers.¹
On the pH scale, citric acid is a weaker acid with a pH ranging between 3 and 6.¹
In 1784, a Swedish chemist named Carl Wilhelm Scheele first extracted citric acid from lemon juice by fermenting molasses or cane sugar with the help of a fungus (Aspergillus niger).²
Citric acid comes as a white fine grain powder. It belongs to the group of carboxylic acids (or organic compounds) that are found in most plants and animals.²
You’ll find commercially produced citric acid in some grocery stores near the home canning supplies. You can also find it online on Amazon, and at most major bath and soap suppliers.

What’s citric acid used for?
Citric acid has a surprisingly wide range of applications. Here are its primary uses:
- Homemade soap: When added to handmade cold process soap recipes, it acts as a chelating agent which neutralizes metal ions in hard water and reduces the appearance of soap scum. It also prevents oxidation in soap oils.
- Home canning: Commonly used in home canning recipes as an acidifier or preservative.
- Commercial foods and drinks: A spritz of citrus enhances the flavor of beverages and commercially produced foods to add a mouth-puckering taste.
- Cosmetics and skincare products: When used in skincare, citric acid provides many benefits: it brightens the complexion, fades dark spots, and reduces wrinkles. The salt of citric acid, sodium citrate, acts as a preservative in personal care products and may adjust the pH level.¹
- DIY bath products: Citric acid puts the fizz in bath bombs when combined with baking soda. It also makes bath salts sparkle.
- Cleaning products: Perhaps it’s no surprise that something derived from lemons, a mainstay in natural cleaning, is also a great cleaning agent in both commercial and homemade products. Citric acid helps remove stains, and leave dishes sparkling clean (no build-up from hard water). Just be careful when using cleaners with high levels of citric acid—it may corrode some metals.
Citric acid + black mold
What’s black mold got to do with it? A lot apparently, when it comes to producing citric acid.
Today, roughly 99 percent of global manufacturing of this zesty acid is used with the help of black mold, also known as the Aspergillus niger fungus.¹
But don’t worry—this practice is generally recognized as safe (GRAS) as deemed by the FDA’s Food, Drug, and Cosmetic Act.¹
Benefits of adding citric acid to homemade soap
Do you need to add citric acid to your soap recipe? Nope, not at all.
You can still make perfectly fine bars of soap with no citric acid at all. If you live in an area with soft water, you might not experience any soap scum at all (lucky you!).
But, if you have hard water, you might want to read up closely in the next sections on how to use citric acid in soap making.
Reduces soap scum
If you’re tired of scrubbing white film off your shower or tub, citric acid can help!
Here’s a quick overview of how soap scum forms and how sodium citrate (the salt of citric acid) in soap helps to reduce it.
If you have hard water, your water contains higher levels of minerals such as magnesium and calcium. When calcium in water joins with soap, it produces a hard white film you know and detest—soap scum.³
When you add citric acid to natural soap recipes using sodium hydroxide lye (NaOH), it produces sodium citrate. Sodium citrate acts as a chelating agent which works to round up metal ions in water and prevent them from getting cozy with soap.
Think of chelators as sticky binders that work to attract metal ions and keep them apart from soap.

Prevents oxidation of oils in soap
If you would like your soap bars to last longer (and who doesn’t?), one way to extend the shelf life is to add a bit of citric acid.
Several things cause those dreaded orange spots (DOS), but sodium citrate is one additive that may help to reduce DOS.
The soap scientist Kevin Dunn did an experiment on various soap additives and how effective they were in preventing DOS.
He found that a combination of both butylated hydroxytoluene (BHT) and sodium citrate worked best to reduce the appearance of those dreaded spots.⁴
Enjoy more lather
If you’re accustomed to low lathering soaps and shampoos because of hard water, you’ll find your soap will lather better than before.
While not every soap will produce big bubbles (depending on the type of oils used), you’ll notice more lather, whether it’s creamy or bubbly.
This is because the sodium citrate works sort of like a water softener to diminish the effects of metal ions which reduce lather.
Common misconceptions about citric acid in soap making
When you hear the terms “pH balanced” or “pH neutral” soaps, people are referring to commercially produced soaps made using synthetic detergents.
If you’re making natural soap using either sodium hydroxide lye (or potassium hydroxide), the bars of soap you make are firmly in the alkaline (or basic) category. Most homemade soaps have a pH level ranging from 8 to 10, with some having higher levels, depending on the ingredients used.
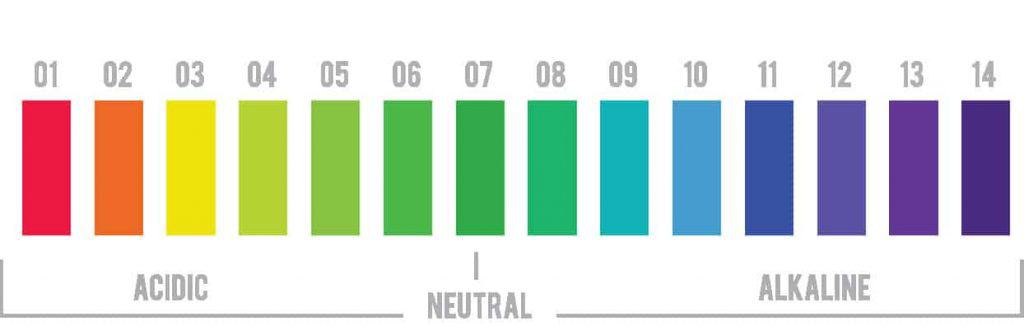
If you recall, the pH scale runs from 0 to 14. A pH of 7 is neutral. Anything lower than pH 7 is acidic. A pH of 0 is super acidic (think battery acid). Anything above pH 7 is considered alkaline or basic (lye has a pH of 13.5).
Citric acid in soap making does NOT effectively lower the pH level in the finished soap.
This is because natural soap is an alkali salt of fatty acids. It must be alkaline in order for it to work like soap.
Adding more citric acid to your soap recipe will not create a pH neutral soap. You’ll end up with a gooey mess! Citric acid displaces some lye, creating a lye discount/increased superfat.
In another science experiment conducted by Dunn, he studied whether it’s possible to notably lower the pH of alkaline homemade soaps through “forced acidification.”⁵
He tested four types of single oil soaps (coconut oil, castor oil, palm oil, and olive oil). One piece was placed in a sealed bag; the second was cured in normal air; the third was placed in a bag filled with carbon dioxide gas; and the last was stored in a bag that held a cup of vinegar.
He concluded that there were negligible changes to the cold process soap’s pH level after exposure to the acidic element (vinegar), and that it’s very difficult to adjust the pH of cold process soap.
Note: That bottle of pH neutral facial cleanser you bought from the drugstore can only achieve pH neutrality through the use of synthetic detergents or surfactants such as sodium lauryl sulfate (SLS).
Citric acid safety
Citric acid in concentrated powder form may irritate skin and eyes. Always wear gloves and protective goggles when working with citric acid, and work in a well-ventilated room.
When added to a lye water solution, citric acid can get very hot, and release fumes (avoid breathing in the fumes). This is a normal reaction whenever an acid reacts with a base (such as sodium hydroxide lye or potassium hydroxide lye).
Treat citric acid as any other chemical or organic compound—with care and caution.
When and how to add citric acid in soap
As citric acid comes in crystal or powder form, you need to first dissolve it in distilled water.
The most common time to add citric acid to your recipe (to my knowledge, anyway) is when preparing your lye solution.
Remember that citric acid will “eat” some of the lye up, so you’ll need to readjust for this in your calculations. Not accounting for extra lye will result in a highly superfatted soap which may be more prone to oxidation, and just plain leave you with a greasy mess.
Here’s a basic formula to keep in mind:
Total lye in recipe = Lye for saponification + Extra lye to neutralize citric acid
Keep in mind that adding citric acid to a base will cause a reaction, so add it slowly and carefully! And put your safety gear on!
- Measure distilled water according to your recipe and place in a heat-safe jug.
- Slowly add citric acid to water and stir until dissolved.
- Carefully add lye flakes to water and stir. Be careful—your solution will get very hot and release fumes!
Tip: There are two types of citric acid: anhydrous and monohydrate. Look for anhydrous citric acid, which does not contain water and is preferable for making soaps and cosmetics.
How much citric acid to add to soap?
There’s no rule written in stone, but most soapers use between 1 and 3 percent citric acid of the total weight of oils.
Adding citric acid to soap requires some math, so put on your thinking cap and break out that calculator!
👉 Here’s what you need to figure out:
- How much lye will you need in your recipe to saponify your oils and fats?
- How much citric acid (in grams or ounces) are you planning to use?
- Calculate the lye needed to neutralize the citric acid in your recipe.
Here are some helpful guidelines. The following numbers apply to anhydrous citric acid only (which is the type most soap/bath suppliers sell).
For sodium hydroxide lye (NaOH):
1 gram citric acid neutralizes 0.624 grams of NaOH. Or, this equates to 10 grams of citric acid required to neutralize 6.24 grams of NaOH.
If you’re adding 1 percent of citric acid, this works out to 10 grams citric acid per every 1,000 grams of oils/fats/butters. You’ll need to add 6.24 grams of extra lye per every 10 grams of citric acid.
Keep in mind that the amount needed to neutralize potassium hydroxide lye is different.
For potassium hydroxide lye (KOH), 10 grams of citric acid neutralizes to 8.42 grams of KOH.
How to store citric acid
For a long shelf life, keep your jar or bottle of citric acid tightly sealed. Keep it in a cool, dry place away from heat sources or direct sunlight.
Related questions
What’s the difference between citric acid and stearic acid in soap making?
The main difference between citric acid and stearic acid in soap making is that the former is mostly used to reduce soap scum, while the latter is used to harden soap.
Stearic acid in soap helps to create a harder bar of soap. There are many fats and oils that may increase the stearic acid content, like hydrogenated soybean oil, kokum butter, and illipe butter. Or, you could also use a bit of pure stearic acid.
What are some natural sources of citric acid?
Citrus fruits, some vegetables, and citrus juices all contain varying levels of citric acid. Of course, we know that lemons and limes contain high amounts of citric acid.
To a lesser degree, you’ll also find citric acid present in most types of berries (but not blueberries), broccoli, carrots, grapefruits, pineapples, tomatoes, and some types of peppers.¹
New to making soap? 🧼❓
👉We have a fantastic overview on the whole soapmaking process here: read our Timeless Guide To Soapmaking.
If you would like to see our soapmaking posts organized by topic type, see our Soapmaking Collection.

References
- Chemical Safety Facts, Citric Acid, https://www.chemicalsafetyfacts.org/citric-acid/. Accessed on December 2021.
- Britannica, Citric Acid, https://www.britannica.com/science/citric-acid. Accessed on December 2021.
- Dunn, Kevin (10 February 2016). “If the World Hands You Lemons,” Wholesale Supplies Plus. Accessed on December 2021.
- Dunn, Kevin (2005), “The Dreaded Orange Spot,” Handcrafted Soap & Cosmetic Guild. https://www.soapguild.org/how-to/make-soap/dreaded-orange-spot.php. Accessed December 2021.
- Caveman Chemistry, http://cavemanchemistry.com/HscgAlkali2015.pdf. Accessed December 2021.

Author: Josh Tesolin
Josh is co-founder of RusticWise. When he’s not tinkering in the garden, or fixing something around the house, you can find him working on a vast array of random side projects.










