What’s a Strong Alkaline Cleaner? 6 Examples and When To Use Them
When you have baked on grease, clogged drains, or dirty surfaces, it’s time to break out an alkaline cleaner.
Alkaline cleaners include soluble salts ranging from mild (baking soda) to strong (washing soda). A commercial alkaline product may also contain water softeners and detergents to boost cleaning power.
Alkaline (aka basic) cleaners are those with a pH above 7 (with 7 being neutral). A strong alkaline cleaner is one with a higher pH with powerful grease and fat removing properties—think oven cleaners or drain cleaners. Use caution when handling strong alkaline cleaners as they are caustic and can harm eyes and skin, and should not be inhaled.
Let’s take a deep dive into the world of strong alkaline cleaning agents, how to use them effectively, and how they differ from acidic cleaning agents. Plus, we have 2 easy DIY recipes below on making your own alkaline cleaner.
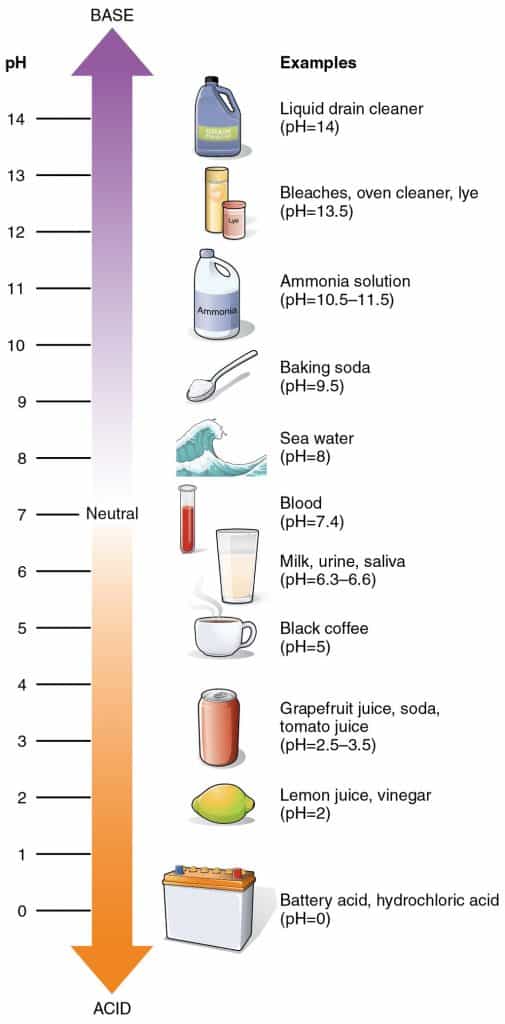
A quick look at the pH scale and how it affects cleaning
Here’s a quick overview of the pH scale.
The pH scale runs from 0 to 14, with pH 7 being neutral. Anything below pH 7 is acidic, and any level above means the substance is alkaline or basic.
The more acidic a solution is, the lower its pH value. Conversely, the more basic a solution, the higher its pH value. For example, a powerful acid is battery acid, which has a pH of 0. On the other end of the scale, sodium hydroxide lye is a strong base, with a pH of roughly 13.5.
Note that a change in number represents a 10-fold change in the alkalinity or acidity of the solution. For example, an alkaline cleaning solution with a pH of 10 is ten times more alkaline than a cleaner with a pH of 9.¹
How does an acid cleaner differ from an alkaline cleaner?
No cleaning solution will be effective against all types of dirt, fat, grease, oil, or mineral buildup. That’s why it’s important to understand the chemistry behind various household cleaners and how they work.
Let’s take a closer look at the differences between acids and bases for cleaning.
In general, acids are useful for removing water buildup, and mineral deposits such as limestone and calcium. Use an acidic cleaner to do the following:
- Remove rust on metal.
- Descale hard mineral deposits from kettles and coffee pots.
- Cut through grease (lemon juice and vinegar).
- Remove hard water deposits on tile and tub.
- Polish metal faucets on sinks, showers, and tubs.
In general, alkalis are useful for fighting fat and grease. Use an alkaline cleaning agent to do the following:
- Remove baked on fat, oil, or grease particles.
- Unclog drains (most particles are oily in nature).
- Remove dirt on surfaces.
- Absorb odors (baking soda, borax, and washing soda are all deodorizers).
- Clean food spills and everyday messes (all-purpose cleaners, soaps, and detergents).
And, let’s not forget about mild soaps, sometimes referred to as neutral washing agents.
Liquid dish soap and Castile soap are considered mild or neutral soap which is gentle enough to clean a wide variety of surfaces.
What are strong household alkaline cleansers used for?
Sometimes messes are so stubborn or severe that you require something with more power. When a sprinkle of baking soda and hot water just isn’t cutting it, it’s time to break out the big guns: heavy-duty cleaners.
A few situations that may call for a strong alkaline agent include:
- Severely clogged drains that aren’t responding to other (milder) clearing methods, such as dish soap, hot water, borax, or a plumbing snake.
- Filthy ovens that have years of baked on grease, oil, and stuck on food particles.
- Heavy dirt buildup on surfaces.
- Cleaning heavily soiled exterior surfaces such as concrete (which responds better to strong base solutions rather than acidic ones.)
- Removing stubborn protein-based soils (such as casein from milk products). Cleaners high in alkalinity are often used in the food industry to clean equipment.²
Keep in mind that cleansers high in alkalinity are corrosive and can damage surfaces with regular use. Reserve powerful alkalis only when needed.
Takeaway: When cleaning at home, it’s best to customize the strength of the cleaner to the severity of the mess. For day-to-day cleaning, start with mild soaps, all-purpose cleaners, and mild alkalis such as baking soda first. Using a very strong alkali to clean a simple spill would be overkill.

Safety tips when using a strong alkaline cleaner
All strong alkalis are toxic if ingested. Please take the proper safety precautions when handling powerful cleaning substances.
- Work in a well-ventilated area and avoid inhaling products.
- Store all products out of reach of young children.
- Wear proper safety gear, including rubber gloves and eye protection. Most heavy-duty cleaners are corrosive and may burn eyes, skin, and harm the respiratory tract.
- Read and follow the instructions on the label. Some products may damage certain surfaces:
- Soft metals such as aluminum, brass, and copper may discolor or corrode. These metals may develop a cloudy appearance with prolonged use.²
- PVC pipes may get damaged from the heat generated when using powerful drain cleaners.
- Porous wood surfaces.
- Rubber components and plastics.
- Fabrics and textiles.
6 examples of strong alkaline cleaners
Table: A Comparison of Mild, Moderate and Strong Alkaline Cleaners
Here’s a quick comparison of various household products grouped according to their alkalinity.³
| Mild Alkalis | Moderate Alkalis | Strong Alkalis |
|---|---|---|
| Baking soda (sodium bicarbonate) | Ammonia | Drain cleaner |
| Dish soap | Borax (sodium borate) | Lye |
| Castile soap | Tri-sodium phosphate (TSP cleaner) | Chlorine bleach |
| Laundry detergent | Oven cleaner | |
| All-purpose cleaners | Washing soda (sodium carbonate) | |
| Glass cleaners | Some concrete cleaners |
- Drain cleaner: Chemical drain cleaners are highly caustic and corrosive. A main active ingredient in drain cleaners is sodium hydroxide lye. Only use chemical drain cleaners as a last resort for severely clogged drains. With repeated use, this chemical cocktail can eat away not only at clogs, but also at the pipes themselves.
- Lye: Lye, also called caustic soda, comes in two forms: sodium hydroxide lye (NaOH) or potassium hydroxide lye (KOH). Most commercial drain and oven cleaners use lye as a base ingredient. If you have a bottle of pure lye at home, you can use it to make homemade soap bars! Please read up on lye safety as lye creates a very strong exothermic reaction (gets boiling hot!) when added to water.
- Chlorine bleach: A bottle of bleach is a strong alkaline solution that’s made by combining chlorine and sodium hydroxide.⁴ The active ingredient in bleach is a sodium hypochlorite solution which provides its potent cleaning ability. Bleach removes organic compounds and stubborn messes such as tea stains and sweat stains to make fabrics bright. Only mix bleach with water. Mixing with other cleaners such as ammonia or vinegar will release toxic fumes!
- Oven cleaner: If you have a self-cleaning oven, don’t even think of using a bottle of commercial oven cleaner! Chemical-based oven cleaners are highly caustic and only intended for traditional ovens. Most oven cleaners contain lye which helps remove heavy greases caked onto oven doors and surfaces.
- Washing soda (sodium carbonate): Sodium carbonate is a naturally occurring mineral mined from the earth. It is the strongest of the three mineral salts (which include baking soda and borax). Take care when using washing soda as it can irritate eyes and skin, and should not be inhaled. Sodium carbonate is a common ingredient in commercial laundry detergent, dishwasher detergent, and other household cleaners.
- Some concrete cleaners: It’s important to note that some (not all) concrete cleaners are strong alkalis. Depending on the type of stains or dirt buildup, as well as the material of the concrete, your concrete may benefit from a strong base solution. Other concrete cleaners may be acidic, or neutral. However, most types of concrete are acid-sensitive, which makes a potent alkali solution effective.

Credit: Yay Images
A closer look at strong alkaline cleaning agents
Most commercial strong alkalis use sodium hydroxide lye (also called caustic soda), or potassium hydroxide lye (aka caustic potash) as a base ingredient.
For example, drain cleaners contain lye. And as you may know, lye + fats/oils = soap.
Using lye in heavy-duty drain cleaners serves as an alkaline builder to convert oil and fat particles (the source of most drain clogs) into soap. The soap then washes down the drain, which helps to clear up the clog as well as dissolve any other debris.
In medium-strength basic solutions such as tri-sodium phosphate (TSP), the active ingredients include sodium, potassium, or ammonium salts of phosphates, silicates, or carbonates such as sodium carbonate. These work to inhibit corrosion, and prevent the buildup of calcium and magnesium in water.²
Don’t forget to rinse well!
As most heavy-duty cleansers may damage surfaces with regular use, it’s important to thoroughly rinse all surfaces with water and dry. This helps keep any potential damage to a minimum.
How to make your own DIY alkaline solution
While there are plenty of commercial cleaners on the market today, you can make your own simple alkaline solution using natural mineral salts. You’ll not only cut down on chemical use at home, these solutions are budget-friendly.
Of the natural mineral salts, baking soda is the mildest, followed by borax, and washing soda is the strongest.
DIY fridge cleaner
Use this simple, effective, and non-toxic alkali solution to clean stainless steel and/or chrome surfaces. It’s perfect for most refrigerators!⁵
You’ll need:
- 2 tablespoons baking soda
- 1 quart (4 cups) warm water
- Optional: spray bottle
Add baking soda to a spray bottle. Mix until dissolved. Use a soft cloth to clean the interior and exterior of fridge. Alternatively, you can create a baking soda paste with just a touch of water to scrub out troublesome spots. Use the baking soda box as a fridge freshener.
DIY toilet bowl cleaner with borax (moderate-strength)
Borax is a medium-strength alkali that’s mildly abrasive—it snuffs out stubborn stains better than baking soda.⁵
You’ll need:
- 1/2 cup borax
- 1 gallon hot (not boiling) water
Sprinkle borax into the toilet bowl, targeting stains. Let it sit for 30 minutes, or longer if needed. Pour the hot (not boiling) water into the bowl. Scrub with brush and flush. (Avoid using boiling water as it may crack the porcelain.)
Related questions
Is vinegar an alkaline cleaner?
No, vinegar is a mild organic acid with a pH of 2–3. It contains acetic acid, which gives this clear liquid its cleaning power. Use this acidic agent to cut through grease, as a rust remover, to descale kettles, and to gently disinfect.
While vinegar is a popular natural and non-toxic ingredient in the green cleaning world, you should still use care when using it. Avoid using vinegar on porous stone countertops and on painted or sealed wooden surfaces.
Is bleach an alkaline cleaner?
Yes, with a pH of roughly 13.5 bleach is a strong alkaline disinfectant. With its ability to keep harmful bacteria, viruses, and other microbes at bay, liquid chlorine bleach is handy to keep around the house and workplace.
As a stain remover, it’s one of the best. But, since it’s so powerful, it’s best to dilute with water. Be careful when using bleach though because its strong cleaning properties can eat away at many materials, such as clothing and upholstery.
What is a mild alkaline cleaner?
Baking soda is one of the most affordable and easy-to-use alkalis. Simply sprinkle a bit of baking soda onto a damp sponge and wipe down surfaces. Rinse well.
Other good mild cleaners that work to remove oily soils, food residue, and for multipurpose cleaning are dish soap and Castile soap.
👉 If you like this post, see other Timeless Cleaning Tips You Need To Know. 🌟
Would you like more timeless tips via email?
Fun tips to help you live an independent, self-sustaining lifestyle. Opt-out at any time.

References
- U.S. Department of the Interior, pH Scale, https://www.usgs.gov/media/images/ph-scale-0. Accessed May 2022.
- University of Florida, BASIC ELEMENTS OF EQUIPMENT CLEANING AND SANITIZING IN FOOD PROCESSING AND HANDLING OPERATIONS, https://edis.ifas.ufl.edu/publication/FS077. Accessed May 2022.
- Hightower, Nancy (03 March 2022). “Spring Cleaning: Cleaner Alternatives,” University of Arkansas (Division of Agriculture). Accessed May 2022.
- Chemical Safety Facts, Sodium Hydroxide, https://www.chemicalsafetyfacts.org/sodium-hydroxide/. Accessed May 2022.
- University of Arkansas (Division of Agriculture), Clean and Green Homemade Cleaners, https://www.uaex.uada.edu/environment-nature/water/quality/clean-green-homemade-cleaners.aspx. Accessed May 2022.

Author: Josh Tesolin
Josh is co-founder of RusticWise. When he’s not tinkering in the garden, or fixing something around the house, you can find him working on a vast array of random side projects.