A Guide on Stearic Acid in Soap + 15 Oils With High Stearic Content
If you’ve ever made a batch of soap that turned out softer than expected, one reason could be that there wasn’t enough stearic acid in the recipe. Stearic acid in soap helps to create a harder bar of soap with stable, creamy lather. You can bump up the stearic acid content by using certain oils, fats, and butters such as hydrogenated soybean oil, kokum butter, and illipe butter to name a few examples.
Or, you could also add a tiny amount of pure stearic acid to your soap recipe. If you’re tired of having soft bars of soap that melt away, let’s look at what exactly stearic acid does in soap, along with tips on using it.
Let’s get started!
A quick overview of fatty acids in soap
Fatty acids are components of soap that help give the finished product its unique properties. Every kind of oil, fat, or butter has its own distinct fatty acid profile.
As a soapmaker, once you get familiar with fatty acid profiles of the main soap making base oils, you can determine the type of soap you’ll end up with. You can choose to make one that’s highly cleansing, for example, or one that’s gentler on the skin.
Crafting the perfect bar of soap often involves experimenting with a blend of oils, fats, and butters to achieve a bar of soap that’s neither too hard, nor too soft, but just right.
We categorize fatty acids in soap into two groups: saturated or unsaturated.
Saturated fatty acids
A good indicator that a fat is saturated is if it’s solid at room temperature (like coconut oil and palm oil). If you’re looking to make an extra firm bar of soap, saturated fats like stearic acid in soap are great to use as their molecular structures allow them to stack together neatly.
If your soap recipe contains a high saturated fatty acid composition, you’ll make hard bars of soap with powerful cleansing properties and strong lather.¹
Here are the four saturated fatty acids commonly found in soap:
- Lauric acid
- Myristic acid
- Palmitic acid
- Stearic acid
Unsaturated fatty acids
In contrast, most unsaturated fatty acids are liquid at room temperature. Examples include avocado oil, olive oil, safflower oil, and soybean oil.
If your soap recipe contains a high unsaturated fatty acid composition, you’ll get bars of soap that are softer, and more moisturizing. Unsaturated fats have less cleansing power. They produce a milder, creamy lather that’s gentle on the skin.¹
Here are the four main types of unsaturated fatty acids:
- Ricinoleic acid
- Oleic acid
- Linoleic acid
- Linolenic acid
👉 Check out our article for more details about fatty acids in vegetable oils.
What exactly is stearic acid?
Stearic acid is a type of long-chain saturated fatty acid with 18-carbon chain (which is why it’s also known as Octadecanoic Acid). You’ll find this naturally occurring fatty acid in both animal fats (lard and tallow) and vegetable oils and butters (coconut oil and palm oil). It’s often a mixed triglyceride with other long-chain fatty acids, and is an ester of a fatty alcohol.²
The word stearic or stearate comes from the Greek work stéar, which means tallow.³ Sodium stearate is the fatty acid salt you’ll find in soaps made using sodium hydroxide lye.
You’ll find that stearic acid is used for many commercial applications: it’s a main component of shaving creams; acts as a thickener and stabilizer in cosmetics, skincare, and other household items; improves viscosity; and acts as a lubricant for industrial purposes.³
At room temperature, stearic acid has no color and is a waxy solid that’s practically insoluble in water.
The formula for stearic acid is: C₁₇H₃₅COOH
Here’s the chemical structure of stearic acid:
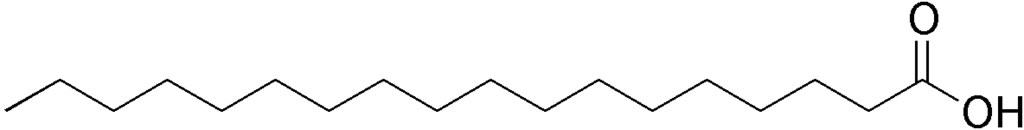
Credit: Wiki Commons
Note: Most stearic acid you’ll find from soap suppliers is derived from palm oil. (We know that not everyone is a fan of palm oil because of its link to environmental issues.) It’s made through the process of hydrolysis and then distilled and pressed. Stearic acid produced in this way is considered vegan.
What does stearic acid do in DIY soap and other personal care products?
If you’re making homemade soaps. lotions, and candles, here’s what stearic acid does:
- Hardens bar soap (cold process, hot process, and melt and pour). Your soaps will release from the mold easier.
- Provides a more stable and creamy lather in bar soap.
- Emulsifies and thickens lotions, creams, body scrubs, and liquid body washes. You’ll find you won’t need to shake your homemade liquid lotions to mix them before use.
- Hardens homemade candles and bumps up the melting point.
15 Soap making oils high in stearic acid
Want to bump up the stearic acid in your soap batch? Consider using one or more of these soap making fats.
While fully hydrogenated soybean oil (aka soy wax) contains the highest levels of naturally occurring stearic acid to my knowledge, it can be a bit tricky to work with. You’ll need to soap at higher temperatures and ensure the wax is fully melted to avoid getting stearic spots.
Many people find that incorporating a butter (or a combination of butters) is an easier way to boost the stearic acid composition in bar soaps.
Tip: When formulating your cold process or hot process soap recipe, a good guideline is to keep the stearic acid content anywhere from 5 to 15 percent. This should provide a reasonably hard bar of soap.
Here are a 15 oils, fats, and butters that contain high to moderate levels of stearic fatty acids.⁴ You’ll notice there are many butters on this list (some with names that I won’t even pretend I know how to pronounce).

Credit: 123RF.com
- Soybean oil, fully hydrogenated (aka soy wax): 87 percent
- Kokum butter: 56 percent
- Illipe butter: 45 percent
- Kpangnan butter: 44 percent
- Sal butter: 44 percent
- Mango seed butter: 42 percent
- Shea butter: 40 percent
- Cupuacu butter: 35 percent
- Cocoa butter: 33 percent
- Ucuuba butter: 31 percent
- Tallow (goat): 30 percent
- Mango seed oil: 27 percent
- Tallow (deer): 24 percent
- Tallow (beef): 22 percent
- Mowrah butter: 22 percent
How much stearic acid to add to cold process or hot process soap
When using store bought stearic acid, less is more. Start off low at around 0.5 percent (of the total oils) in cold process or hot process soap making. You can bump it up to a max of 1–2 percent of the recipe.
Adding too much may cause false trace.
Tips on working with stearic acid in soap
Working with stearic acid in soap is tricky. Stearic acid reacts quickly sometimes producing false trace. It also has a fairly high melting point which requires you to soap at higher temperatures.
Note: The melting point of stearic acid is 156.7 degrees Fahrenheit (69.3 degrees Celsius).
Here are a few tips on working with this finicky additive.
Related questions
What are stearic spots?
Stearic spots are tiny white dots that are visible in finished soap. They can occur when using oils or fats high in stearic or palmitic acid and soaping at lower temperatures. It’s important to fully melt any hard oils first before combining liquid oils.
The good news is that in most cases, soap with stearic spots should be safe to use. (They might not look picture-perfect, but that’s okay!)
How much stearic acid to use in melt and pour soap?
Start with a low amount: 0.5 percent of total oils. Depending on your melt and pour recipe, you could add as much as 3 percent.
If you decide to add a bit of stearic acid to melt and pour soap, it’s best to fully melt your soap bases and stearic acid separately before combining.
New to making soap? 🧼❓
👉We have a fantastic overview on the whole soapmaking process here: read our Timeless Guide To Soapmaking.
If you would like to see our soapmaking posts organized by topic type, see our Soapmaking Collection.

References
- Grosso, Alicia (2016). DIY Artisanal Soaps: Make Your Own Custom Handcrafted Soaps! Adams Media. pp. 179-180. ISBN 978-1-4405-9408-3.
- Britannica, Stearic acid, https://www.britannica.com/science/stearic-acid. Accessed December 2021.
- American Chemical Society (ACS), Sodium stearate, https://www.acs.org/content/acs/en/molecule-of-the-week/archive/s/sodium-stearate.html. Accessed December 2021.
- Soap Calc, Oil List, http://www.soapcalc.net/calc/OilList.asp. Accessed December 2021.

Author: Josh Tesolin
Josh is co-founder of RusticWise. When he’s not tinkering in the garden, or fixing something around the house, you can find him working on a vast array of random side projects.